HEOR and Market Access Strategies in Today’s Drug Development Landscape – Insights from AstraZeneca’s Dr. Heather McDonald
XTalks
OCTOBER 4, 2023
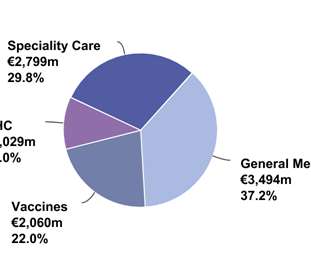
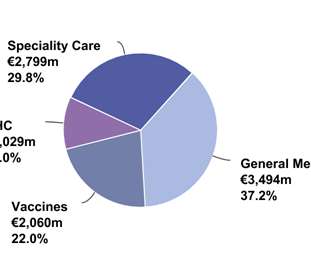
In my current role, I have country-level responsibility for AstraZeneca’s entire portfolio, including cardiovascular, respiratory, vaccines and oncology. In the specialized, personalized and advanced medicines spaces, this work should start very early — as early as Phase I or Phase II of clinical development.








































Let's personalize your content