Valneva’s Lyme disease vaccine faces final clinical test in a sparse landscape
Pharmaceutical Technology
AUGUST 17, 2022
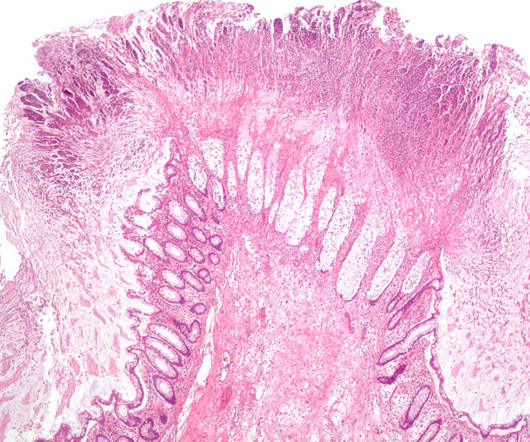
Lyme disease is also known as borreliosis, which refers to the borrelia bacteria that cause the condition. A vaccine called Lymerix , then manufactured by SmithKline Beecham, was approved in the US in 1998 but was quickly withdrawn in 2002 due to insufficient custo mer demand. It is currently treated with antibiotics.
































Let's personalize your content