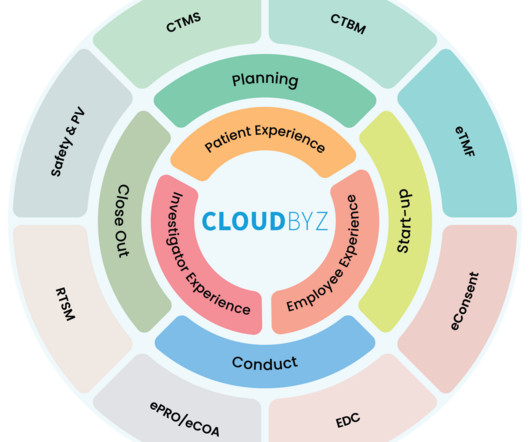
Why a Platform Approach is Vital for Clinical Research Management Transformation
Cloudbyz
AUGUST 9, 2023
The clinical research landscape is rapidly evolving. As it becomes more complex with growing volumes of data, evolving regulations, and the pressure for faster drug development, traditional methods of clinical research management are no longer sufficient. This is where the platform approach comes into play.
































Let's personalize your content