Tech innovations that curb complexity, setting clinical trials up for success
Pharmaceutical Technology
OCTOBER 26, 2022
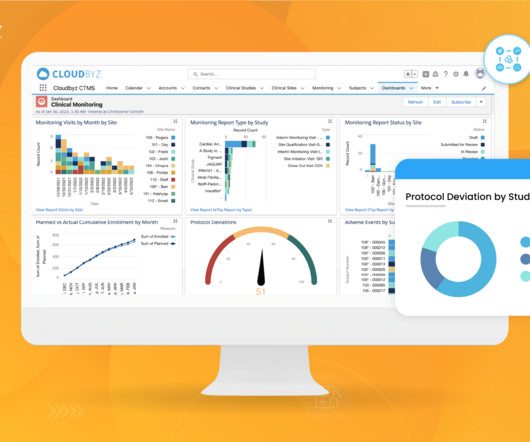
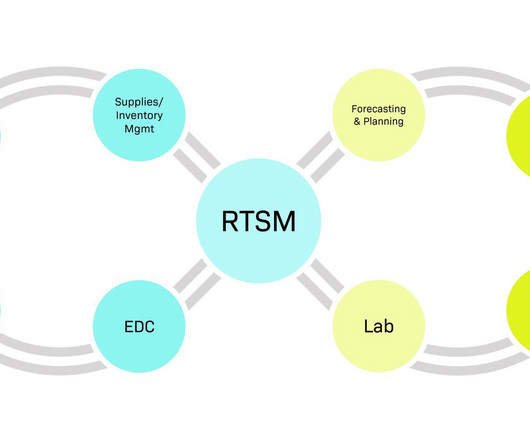
There is no denying that trial complexity is continuing to increase. Several factors in recent years have contributed to this rise including more complex trial designs, the rapid adoption of decentralisation stemming from the pandemic, and larger global trials. Demand planning drives trial efficiency.












































Let's personalize your content