Changing cell & gene therapy landscape and implications on clinical trials
Bio Pharma Dive
APRIL 3, 2023
Cell & gene therapy clinical research may help treat diseases and change how we approach healthcare.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
Bio Pharma Dive
APRIL 3, 2023
Cell & gene therapy clinical research may help treat diseases and change how we approach healthcare.
Pharmaceutical Technology
MAY 24, 2023
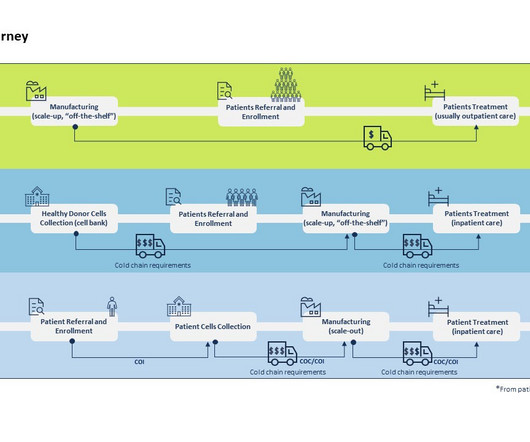
By Luisa Sterkel & Joana Loureiro , Tenthpin Consultants The promise and potential of cell and gene therapies (CGT) has emerged in the recent past and currently over 1.500 CGT are registered for clinical trials holding great hope for the treatment of challenging and uncurable diseases.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
FEBRUARY 8, 2023
On February 7, at a town hall organised to discuss clinical trial designs for gene therapies, FDA experts pushed pharma players to look for ways to establish clinical effectiveness despite the challenges in recruiting patients with rare diseases.

pharmaphorum
FEBRUARY 27, 2024
Looking to the future of clinical trials: Gene therapy, precision medicine, and the ongoing quest for rare disease solutions Mike.Hammerton Tue, 27/02/2024 - 12:30 Bookmark this

XTalks
MAY 17, 2023
The Foundation for the National Institutes of Health (FNIH) announced this week that the Accelerating Medicines Partnership Bespoke Gene Therapy Consortium (AMP BGTC) has selected eight rare diseases for its clinical trial portfolio.
Bio Pharma Dive
MAY 17, 2022
An unusual collaboration among gene therapy developers suggests certain mutations could be behind "peculiar" side effects experienced by several patients treated in clinical trials.

Pharmaceutical Technology
MAY 3, 2024
The FDA has awarded the designation following a review of initial safety and efficacy data from two Phase I/II clinical trials.
Bio Pharma Dive
JULY 22, 2021
Three companies are testing their gene therapies in early clinical trials, with initial results due later this year. Others are in preclinical stages and aim to follow soon.

Bio Pharma Dive
MAY 16, 2023
Backed by nearly $100 million, the public-private consortium aims to create a standard development roadmap for gene therapies using AAV viral vectors.

Bio Pharma Dive
DECEMBER 11, 2023
Editas had the tall task Monday of convincing ASH attendees its gene therapy for sickle cell disease could improve on Casgevy and Lyfgenia.

Pharmaceutical Technology
NOVEMBER 8, 2022
Cyagen and Neurophth Therapeutics have entered a strategic partnership to jointly develop next-generation AAV gene therapy vectors for specific kinds of genetic ophthalmic ailments. Additionally, Neurophth will oversee the clinical trials and marketing of gene therapy products developed leveraging the new AAV capsids of Cyagen.

Bio Pharma Dive
APRIL 4, 2022
Safety issues in clinical trials cast doubt on the ability of gene therapy to safely mitigate disease, prompting further research into understanding the virus-host interaction that resulted in new generations of viral vectors.

Bio Pharma Dive
OCTOBER 4, 2021
Initiation of the Phase 3 trial is an important milestone for the biotech after earlier setbacks, as well as for patients with the inherited muscle disease.

Bio Pharma Dive
JULY 7, 2022
A year after reporting side effects “not seen before in ocular gene therapy” in a clinical trial, the biotech is eliminating 38% of its workforce to save cash while it runs a new study.
Pharmaceutical Technology
JUNE 13, 2023
Beacon Therapeutics has kickstarted its entry into the gene therapy field with a $120m Series A financing. Amongst it was AGTC’s lead clinical candidate, AFTC-501, an adeno-associated virus (AAV) gene therapy for XLRP. The asset, now transferred to Beacon Therapeutics, is currently in Phase II clinical trials.

XTalks
JANUARY 8, 2024
Whether it’s for a treatment for a chronic ambulatory condition, precision medicine or cell and gene therapy, there is a massive uptick in clinical trial complexity. The key considerations she shared include: Project management: The expertise and approach of the team managing the trial.

Pharmaceutical Technology
APRIL 3, 2023
The deal will see Polyplus join the German life science group’s portfolio allowing the latter to leverage expertise in transfection reagents and plasmid DNA for gene therapy. Polyplus, based in Strasbourg, France, produces key components in the production of viral vectors used in cell and gene therapies.
Pharmaceutical Technology
OCTOBER 25, 2022
Ast ellas Pharma has announced plans to make a strategic investment to back the development of Taysha Gene Therapies’ adeno-associated virus (AAV) development programmes for Rett syndrome and giant axonal neuropathy (GAN). Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
Pharmaceutical Technology
FEBRUARY 2, 2023
If things go as per plan, in a few months, the US Food and Drug Administration (FDA) will deliberate on the first-of-its-kind CRISPR-based gene therapy for sickle cell disease (SCD) and transfusion-dependent beta thalassemia. We also explore how AI is being used to design digital twins for clinical trials.

Pharmaceutical Technology
NOVEMBER 23, 2022
The US Food and Drug Administration (FDA) has granted approval for CSL Behring’s adeno-associated virus vector-based gene therapy, Hemgenix (etranacogene dezaparvovec), to treat haemophilia B (congenital Factor IX deficiency) in adult patients. The trial is designed to analyse the safety and efficacy of Hemgenix.

BioPharma Reporter
AUGUST 24, 2022
BrainVectis, a subsidiary of AskBio, has received clearance from the French authorities to start a Phase 1-2 clinical trial for its novel Huntingtonâs Disease gene therapy, BV-101.

BioPharma Reporter
JANUARY 18, 2024
The Cell and Gene Therapy Catapult (CGT Catapult), an independent organization specialising in the advancement of cell and gene therapies, has revealed that the UK has remained an attractive destination for commercial trials, with this type of clinical trial accounting for 81% of trials in 2023 and 80% in 2022.

Pharmaceutical Technology
MAY 16, 2023
The Foundation for the National Institutes of Health (FNIH) has announced its plans to prioritise eight rare diseases to provide industry standards for manufacturing, preclinical testing and product analytical testing for gene therapy development. This will include pairing up indications with manufacturers amongst the BGTC’s partners.
Pharmaceutical Technology
MAY 30, 2023
Amplo Biotechnology has received a fast track phase I/II STTR grant from the NIH-NIAMS [National Institutes of Health’s National Institute of Arthritis and Musculoskeletal and Skin Diseases] for its gene therapy AMP-201. The company will receive substantial funding to advance AAV-ColQ gene therapy.

Pharmaceutical Technology
MARCH 10, 2023
On 10 March, the National Health Service Blood and Transplant (NHSBT) opened a new Clinical Biotechnology Centre (CBC) with the aim of improving the UK’s ability to develop and manufacture cell and gene therapies. Personalised medicines will also be developed at the centre.

Bio Pharma Dive
JUNE 27, 2023
A Duchenne gene therapy faces a crucial test, while highly anticipated study results are expected in lung cancer, obesity and heart disease.

BioPharma Reporter
APRIL 4, 2022
The first patient has been dosed in the Phase 1/2 clinical trial of OCU400, a modifier gene therapy candidate for the treatment of retinitis pigmentosa (RP) resulting from mutations in the nuclear receptor subfamily 2 group E member 3 (NR2E3) and Rhodopsin (RHO) genes.

Pharmaceutical Technology
JANUARY 24, 2024
The company could be in line for a priority review voucher if 4D-710, currently in Phase I/II clinical trials, is approved.
Pharmaceutical Technology
AUGUST 25, 2022
The European Commission (EC) has granted conditional marketing authorisation (CMA) for BioMarin Pharmaceutical ’s gene therapy, Roctavian (valoctocogene roxaparvovec), to treat adults with severe haemophilia A (congenital Factor VIII deficiency). Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.

BioPharma Reporter
MARCH 5, 2024
Neurogene, a clinical-stage company developing genetic medicines, is expanding its ongoing phase 1/2 clinical trial investigating NGN-401 as a treatment for female pediatric patients with Rett Syndrome.

Worldwide Clinical Trials
JANUARY 24, 2024
Written By: Derek Ansel, MS, CCRA, Executive Director, Therapeutic Strategy Lead, Rare Disease Given that 80% of rare diseases have a genetic etiology, genetic implications should be addressed at the onset of a clinical program to support trial enrollment. One diagnostic example that I discussed in my presentation is autism.

XTalks
JANUARY 17, 2024
Estimates based on publicly available information suggest more than 40 percent of all new therapies in development are cancer treatments. Given this hotbed of activity, innovation in the space to drive faster decisions and more efficient trials is intense.

BioSpace
NOVEMBER 15, 2023
The Japanese pharma contends that an analysis of the four deaths in its AT132 gene therapy clinical trial shows it is still viable as a potential treatment for a fatal, rare genetic disease.

XTalks
JUNE 14, 2023
Courtney Silverthorn who is an Associate Vice President at the Foundation for the National Institutes of Health (FNIH) and the Director of the Accelerating Medicines Partnership (AMP) program including the Bespoke Gene Therapy Consortium. Subscribe to the Xtalks Life Science Podcast to never miss a new episode.
Pharmaceutical Technology
JULY 7, 2022
For many decades, investigators have been working on innovative therapeutic modalities known as cell and gene therapies, which use modified versions of the body’s own cellular and genetic material to treat and potentially cure these diseases. A new frontier in cancer research.

Camargo
JULY 27, 2021
How and When to Incorporate PK Design into Your Gene Therapy Development Plan. Gene therapy, which was in its infancy around 30 years ago, is now becoming a more prominent treatment method in many therapeutic areas, from personalized therapy to mass vaccinations against COVID-19. Gene Therapy Definition.

XTalks
APRIL 24, 2023
Awareness of rare diseases is growing, and with a better understanding of the pathophysiology of many rare diseases, innovative treatment options are emerging, like gene therapies that can treat the root cause of rare genetic diseases and potentially provide long-term symptom relief, or even a definitive cure.

Pharmaceutical Technology
JULY 22, 2022
The spotlight was placed on many up-and-coming pharmacotherapies for retinal diseases, one of which was AbbVie’s/Regenxbio’s gene therapy RGX-314. Clinical trial results in DR patients have shown that 47% of eyes treated with RGX-314 have experienced diabetic retinopathy severity score (DRSS) improvements of two or more steps.

Worldwide Clinical Trials
JUNE 15, 2022
At the end of May, we hosted a webinar titled “ Changing Times, Changing Therapies: Keeping Up with Advancements in Cell and Gene Therapies ” to provide a quick update on the latest advancements and ongoing in development of these advanced therapeutics. Around 40% of clinical holds are for gene therapy programs.
Bio Pharma Dive
MARCH 19, 2021
Stung by a clinical trial miss in Duchenne muscular dystrophy, the biotech now has promising, if early, data to back a gene therapy for a different rare neuromuscular disease.
Pharmaceutical Technology
JULY 14, 2022
Avrobio has received orphan drug designation for its gene therapy, AVR-RD-05, from the US Food and Drug Administration (FDA) to treat mucopolysaccharidosis type II (MPSII) or Hunter syndrome. The company noted that this gene therapy is the fourth one to receive orphan drug designation.

Bio Pharma Dive
NOVEMBER 28, 2022
The challenges biopharma companies face in running clinical trials and tips on how to surmount those obstacles.
Drug Discovery World
OCTOBER 9, 2023
Positive Phase I trial data indicates that Reqorsa (quaratusugene ozeplasmid) has shown early efficacy for the treatment of non-small cell lung cancer (NSCLC). Developed by gene therapy company Genprex, Reqorsa is a non-viral gene therapy that leads to expression of the TUSC2 tumour suppressor gene in cancers.

Pharmaceutical Technology
MAY 25, 2023
Pushing back an initial deadline, the US Food and Drug Administration (FDA) has proposed a new regulatory action date of 22 June, by which time the agency will assess the logistics of a possible approval for Sarepta Therapeutics’ Duchenne muscular dystrophy (DMD) gene therapy.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content