Clinical Trial Data Archiving: Ensuring Efficiency, Compliance, and Accessibility
Cloudbyz
JUNE 16, 2023
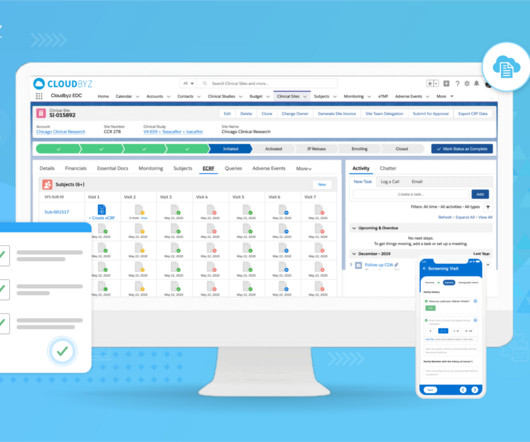
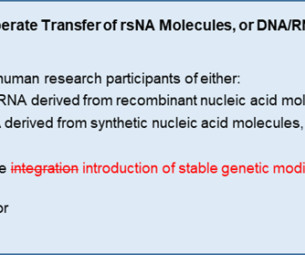
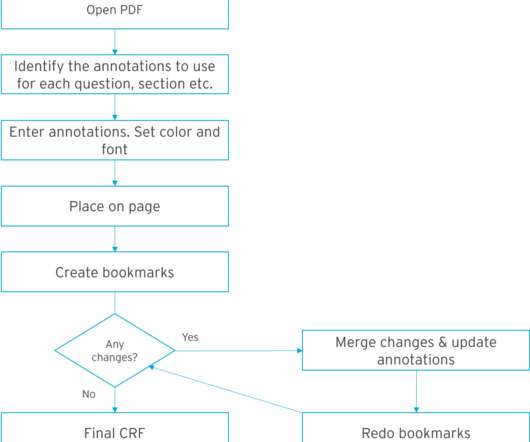
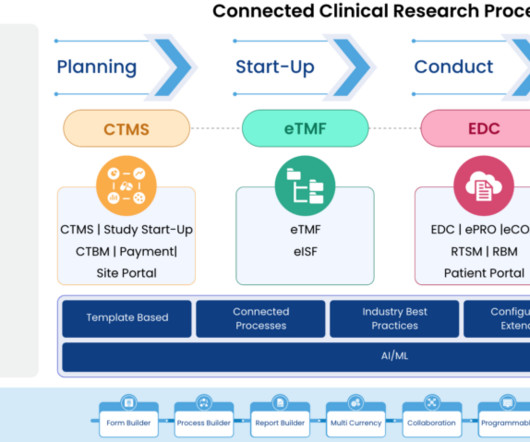
Clinical trials are crucial for advancing medical research and developing innovative treatments. Effective clinical trial data archiving is essential to ensure data integrity, regulatory compliance, and seamless access. Effectively managing and storing such large datasets presents logistical challenges.









































Let's personalize your content