US FDA grants Fast Track status for Avidity’s AOC 1020 to treat FSHD
Pharmaceutical Technology
JANUARY 19, 2023
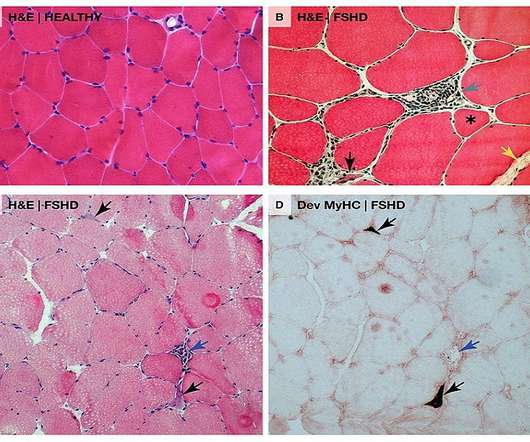
AOC 1020 has been designed for the treatment of the underlying cause of FSHD, which is caused by the abnormal expression of a gene known as double homeobox 4 or DUX4. Avidity Biosciences’ AOC 1020 is intended to reduce the DUX4 mRNA and DUX4 protein expression in muscles in these patients.





























Let's personalize your content