CAMP4’s funding influx paves the way for tapping regulatory RNA to treat urea cycle disorders
Pharmaceutical Technology
JULY 29, 2022
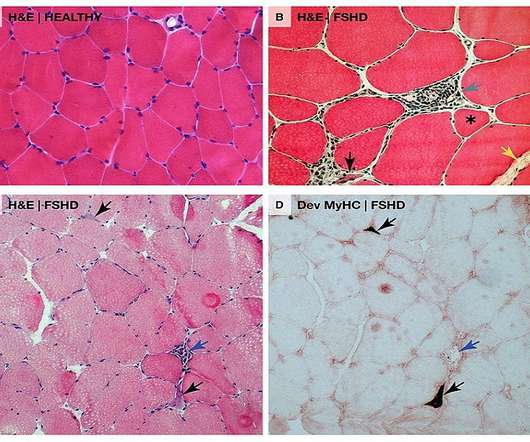
Last week, CAMP4 Therapeutics announced the close of a $100 million Series B round , which will be used to advance their regulatory RNA (regRNA)-focused programs. CAMP4’s CSO David Bumcrot PhD tells Pharmaceutical Technology that the company plans to see clinical trials go forward for their urea cycle disorder programs late next year.

























Let's personalize your content