Biotech startup Structure pulls off rare IPO, raising $161M
Bio Pharma Dive
FEBRUARY 2, 2023
The company’s lead drug could be an oral alternative to marketed diabetes and obesity drugs from Novo Nordisk and Eli Lilly.

Bio Pharma Dive
FEBRUARY 2, 2023
The company’s lead drug could be an oral alternative to marketed diabetes and obesity drugs from Novo Nordisk and Eli Lilly.

Pharmaceutical Technology
FEBRUARY 2, 2023
The US Food and Drug Administration (FDA) has approved GlaxoSmithKline ’s (GSK) Jesduvroq (daprodustat) to treat anaemia caused by chronic kidney disease (CKD) in adults who have been on dialysis for at least four months. Jesduvroq is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI). It is claimed to be the only HIF-PHI approved in the country that offers a new oral treatment option for adult patients on dialysis with anaemia of CKD.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
FEBRUARY 2, 2023
Though Goldfinch Bio focused on kidney diseases, Karuna believes its experimental drugs may have potential treating psychiatric and neurological conditions.
Pharmaceutical Technology
FEBRUARY 2, 2023
If things go as per plan, in a few months, the US Food and Drug Administration (FDA) will deliberate on the first-of-its-kind CRISPR-based gene therapy for sickle cell disease (SCD) and transfusion-dependent beta thalassemia. Having made significant advances in a relatively short period of time, CRISPR research is now edging closer to reaching the clinic , and this month’s cover story takes a look at the major players in this field and the big events that could break through this year.
Bio Pharma Dive
FEBRUARY 2, 2023
The British drugmaker sees potential for the medicine to be a functional cure for chronic infections, and is beginning two Phase 3 trials to test its promise.

AuroBlog - Aurous Healthcare Clinical Trials blog
FEBRUARY 2, 2023
Breathing in air pollution could have an impact on the way your brain is wired, with scientists at the University of British Columbia (UBC) and the University of Victoria discovering that inhaling car exhaust can change a brain’s connectivity within two hours.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
Pharmaceutical Technology
FEBRUARY 2, 2023
Cidara Therapeutics has announced the expansion of its existing collaboration with WuXi XDC for advancing the CD73 oncology DFC programme. The deal builds on the existing partnership with WuXi XDC for chemistry, manufacturing, and controls (CMC) development and GMP manufacturing services for the CD388 influenza drug-Fc conjugate (DFC) programme of Cidara.

Bio Pharma Dive
FEBRUARY 2, 2023
Still, fourth quarter sales for Mounjaro were slightly below high Wall Street forecasts and didn’t offset falling revenue from Lilly’s COVID-19 antibodies.

AuroBlog - Aurous Healthcare Clinical Trials blog
FEBRUARY 2, 2023
The Subject Expert Committee (SEC) that advises the drug regulator of the country has recommended waiver of local phase III and IV clinical trials in India for Sanofi Healthcare India’s orphan drug to treat the Pompe disease. The Committee recommended grant of permission to import and market the drug without these stages of studies.

Bio Pharma Dive
FEBRUARY 2, 2023
Teresa Graham, currently head of Roche Pharmaceuticals’ global product strategy, will succeed former chief Bill Anderson. The reshuffle is one of several recent changes in the pharma’s leadership.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
FEBRUARY 2, 2023
The University of Texas MD Anderson Cancer Center has announced a strategic collaboration with Federation Bio for the development of a new microbiome treatment for immunotherapy-resistant cancer patients. The collaboration is aimed at designing and developing a complex, synthetic microbial consortium to expand the number of cancer patients who respond to immunotherapy.

STAT News
FEBRUARY 2, 2023
Inferior vena cava filters are supposed to save lives. The spider-like devices catch blood clots before they can travel up to the lung and cause deadly pulmonary embolisms. But for over a decade, these devices have been dogged by questions about how well they work and the serious complications they can cause for patients. The latest data make clear they’re still causing problems: Researchers examined a Food and Drug Administration database and found that adverse event reports related to t

NPR Health - Shots
FEBRUARY 2, 2023
Congress ended the temporary benefit meant to help low-income households with pandemic-era hardships. A huge increase in Social Security benefits may mean some households see further SNAP reductions.

STAT News
FEBRUARY 2, 2023
WASHINGTON — A majority of Americans support banning all tobacco products, according to a new poll published by researchers at the Centers for Disease Control and Prevention. The survey , which was published Thursday in the peer-reviewed journal Preventing Chronic Disease, asked 6,455 people nationwide: “To what extent would you support a policy to prohibit the sale of all tobacco products?
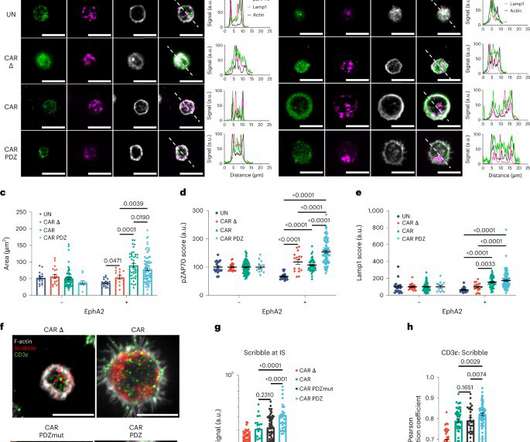
Medical Xpress
FEBRUARY 2, 2023
Adding a molecular anchor to the key protein used to recognize cancer in cellular immunotherapies can make the treatments significantly more effective. Scientists at St. Jude Children's Research Hospital found that immune cells with the anchored protein increased cancer killing, regardless of their cell type or the kind of cancer targeted.
STAT News
FEBRUARY 2, 2023
NEW YORK — U.S. health officials are advising people to stop using over-the-counter eye drops that have been linked to an outbreak of drug-resistant infections. The Centers for Disease Control and Prevention on Wednesday night sent a health alert to physicians, saying the outbreak includes at least 55 people in 12 states. One died.
Medical Xpress
FEBRUARY 2, 2023
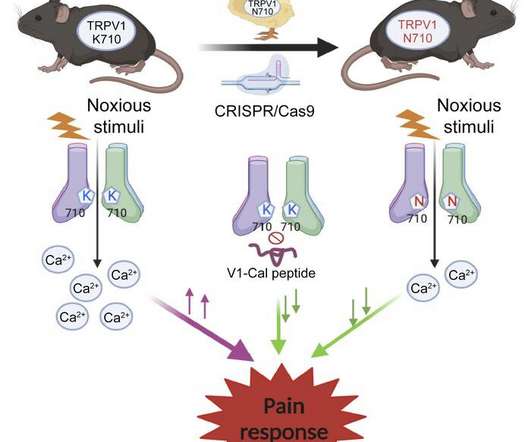
Stanford Medicine researchers have discovered a possible new way to treat pain without the use of opioids. By targeting a specific area of a well-known pain receptor, they were able to reduce pain sensitivity in mice without affecting the receptor's other functions, such as sensitivity to heat.

STAT News
FEBRUARY 2, 2023
WASHINGTON — Teva Pharmaceuticals has quit the Pharmaceutical Research and Manufacturers of America, STAT has learned. “ Teva has decided not to renew its membership with PhRMA in 2023,” PhRMA spokesperson Brian Newell confirmed.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
Medical Xpress
FEBRUARY 2, 2023
An unexpected discovery made by experts at the Brain Tumor Center at Cincinnati Children's while studying a rare-but-deadly type of brain tumor may also lead to improved treatments for several other forms of cancer that share a common influencing factor called a YAP fusion protein.

Rethinking Clinical Trials
FEBRUARY 2, 2023
The NIH Pragmatic Trials Collaboratory has developed an online tool to evaluate the complexity of delivery of a trial intervention. To develop the tool, principal investigators of embedded pragmatic clinical trials (ePCTs) shared critical drivers of complexity that affected their ability to implement an intervention and discern treatment effects. A manuscript describing the tool and its development was published today in Contemporary Clinical Trials.
STAT News
FEBRUARY 2, 2023
The deaths of nearly 30 children under the age of 15 years from invasive strep A infections in the United Kingdom between September 2022 and January 2023, together with a rise in new invasive strep A infections in that country, made headlines around the world. In Kenya, about 100 children aged 5 and younger died from invasive strep A infections over the same period — which did not make headlines.

ACRP blog
FEBRUARY 2, 2023
Changing ‘I fell into this’ into ‘This was my career of choice.’ Many clinical research professionals “fell into” this career pathway rather than choosing it deliberately from the outset. With today’s well-known workforce challenges, it is more important than ever for sites to address workforce development in a meaningful way, reimagining how clinical research departments perform in an organizational context.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

STAT News
FEBRUARY 2, 2023
Is the next Lipitor on the way? When is a pandemic over-over? And what’s a Texas two-step? We cover all that and more this week on “The Readout LOUD,” STAT’s biotech podcast. STAT Washington correspondent Rachel Cohrs joins us to explain the looming end of Covid-19’s status as a federal emergency and what that does and doesn’t mean for public health.

Medical Xpress
FEBRUARY 2, 2023
Electric vehicles are widely hailed as a key way to mitigate climate change through reduced emissions, but research on the dual benefits of reduced air pollution and improved health has been largely hypothetical.

STAT News
FEBRUARY 2, 2023
There are about as many Americans living with addiction as there are Americans living with cancer — but you wouldn’t know it based on the world of venture capital. In the past decade, investment firms have poured roughly 270 times more money into developing cancer drugs than addiction cures, according to a new report from BIO, the biotechnology industry trade group.
Drug Discovery World
FEBRUARY 2, 2023
This is the latest episode of the free DDW podcast. It covers two articles written for Volume 23, Issue 1 – Winter 2021/22 of DDW. They are called “ Why synthetic biology is the next big thing for biopharma ” and “ Collaborative spirit helps drive innovation ”. ‘Can synthetic biology save us?’ and ‘Synthetic biology can benefit us all’, are just some of the headlines this emerging scientific field has recently elicited.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
STAT News
FEBRUARY 2, 2023
China’s massive Covid-19 crisis, sparked in part by the country’s rolling back its strict zero-Covid policies in early December, has seen millions of people infected with SARS-CoV-2. Making the problem worse is that supplies of name-brand Covid-19 drugs are few and far between, and generics of these drugs won’t be available in China due to failed manufacturer negotiations.

Antidote
FEBRUARY 2, 2023
The statistics about the prevalence of cancer can be disheartening. Over 10 million people die each year from cancer , making it one of the leading causes of death — and unfortunately, researchers have yet to find a cure for many cancers.
STAT News
FEBRUARY 2, 2023
An incident that took place at a Dutch polio vaccine production facility late last year is a critical reminder of a major challenge the world faces if and when polio eradication is completed: How do we keep polio from re-establishing itself, given that laboratories and vaccine manufacturers in numerous countries will need to continue to work with the viruses?
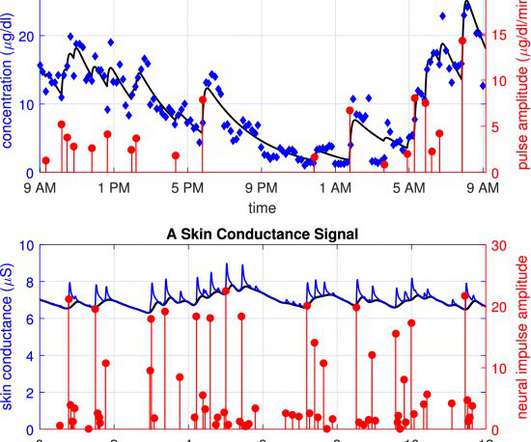
Medical Xpress
FEBRUARY 2, 2023
Wearable monitoring is likely to play a key role in the future of health care. In many cases, wearable devices may monitor our physiological signals that can indicate mental states, such as emotions.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Let's personalize your content