Wilson Bryan, former FDA gene therapy leader, joins consulting firm
Bio Pharma Dive
JULY 19, 2023
The agency veteran, who led the Office of Tissues and Advanced Therapies during a boom in gene and cell therapy research, retired in March.
Bio Pharma Dive
JULY 19, 2023
The agency veteran, who led the Office of Tissues and Advanced Therapies during a boom in gene and cell therapy research, retired in March.

Pharmaceutical Technology
JULY 19, 2023
Twist Bioscience and CRUK innovation arm Cancer Research Horizons have signed an agreement for licensing a library of libraries.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 19, 2023
India’s pharmaceutical market is growing at a healthy rate and is expected to touch 130 billion USD by 2030, said Dr. SV Veeramani, chairman of the Pharmaceuticals Export Promotion Council of India (Pharmexcil) while delivering the keynote address at the Pharmac South Expo 2023 in Chennai on July 14.
Pharmaceutical Technology
JULY 19, 2023
B. pseudomallei is resistant to many antibiotics and, without treatment, can be lethal to those infected.

Bio Pharma Dive
JULY 19, 2023
The pharma claimed in federal court that the Inflation Reduction Act, which threatens sales of its blood thinner Xarelto, amounts to “confiscation of constitutionally protected property.

Rethinking Clinical Trials
JULY 19, 2023
From left: Dr. Christine Goertz and Dr. Adam Goode of the IMPACt-LBP Demonstration Project In this Friday’s PCT Grand Rounds, Dr. Christine Goertz and Dr. Adam Goode of Duke University will present “Implementing New Care Pathways for Low Back Pain in Academic Healthcare Systems: Early Lessons From IMPACt-LBP.” The Grand Rounds session will be held on Friday, July 21, 2023, at 1:00 pm eastern.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
JULY 19, 2023
The follow-on stock offering is particularly large for a biotechnology company and will bolster the financial position of Argenx, now worth nearly $30 billion.

Pharmaceutical Technology
JULY 19, 2023
Amarin Corporation has initiated a company restructuring programme, following a business review by its new board and senior management team.
Bio Pharma Dive
JULY 19, 2023
The two drugmakers moved to trim costs following clinical and commercial setbacks, joining a lengthening list of biotechs forced into similar restructurings.

Pharmaceutical Technology
JULY 19, 2023
The biotech announced its employee cuts as part of a strategic update partly caused by the termination of its AstraZeneca collaboration.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Rethinking Clinical Trials
JULY 19, 2023
This podcast continues the discussion with Dr. Julie Fritz as she discusses partnering with community health centers within a socio-technical framework through BeatPain Utah. Click on the recording below to listen to the podcast. NIH Pragmatic Trials Collaboratory · Podcast 45: From Observational Studies to Pragmatic Clinical Trials: Research in PCORnet® NIH Pragmatic Trials Collaboratory · Podcast 44: The Heartline Trial: A New Paradigm in Conducting Virtual Clinical Trials NIH Pragmatic Tria

Pharmaceutical Technology
JULY 19, 2023
Advent Therapeutics has received a grant worth $3m from the US NIH for expediting the development of bronchopulmonary dysplasia therapy.

Rethinking Clinical Trials
JULY 19, 2023
Speaker Christopher B. Granger, MD Donald F. Fortin, M.D. Distinguished Professor of Medicine Professor of Nursing Duke University Neha J. Pagidipati, MD MPH Associate Professor of Medicine, Duke University School of Medicine Slides Keywords COORDINATE-Diabetes; Cardiovascular disease; Intervention; Cluster-randomized trial; Implementation; Prescription Key Points High-intensity statins, ACEi/ARBs, and SGLT2i and GLP-1RA have been shown to improve recovery and maintenance outcomes for pa

Pharmaceutical Technology
JULY 19, 2023
Novartis has reported net sales of $13.62bn in Q2 of 2023, indicating a 7% rise compared to $12.78bn in the same quarter of 2022.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharma Marketing Network
JULY 19, 2023
Sponsorships can be a powerful tool for pharmaceutical companies to engage with communities and make a positive impact on health and wellness. When done right, sponsorships can help pharmaceutical companies connect with their target audience, build relationships with key stakeholders, and promote their brand in a meaningful way. Here are some examples of how pharmaceutical companies can use sponsorships to engage communities: Sponsoring community health events: Pharmaceutical companies can s
Pharmaceutical Technology
JULY 19, 2023
The Phase I trial evaluates the combination Telix’s targeted radiation therapy with Merck’s peposertib, a DNA-PK Inhibitor.

Pharma Marketing Network
JULY 19, 2023
Content Marketing as a Sales Driver: Unlocking Your Business Potential in the Pharmaceutical Industry Content marketing is a long-term strategy that can help businesses of all sizes generate leads, increase brand awareness, and drive sales. By creating and distributing valuable content, businesses can attract and engage their target audience, position themselves as experts in their field, and build trust with potential customers.

Pharmaceutical Technology
JULY 19, 2023
The agreement covers licensing rights in North America for Duchenne muscular dystrophy and other future indications.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharma Marketing Network
JULY 19, 2023
Sponsorships can be a powerful way for businesses to connect with their target audience, build relationships with key stakeholders, and promote their brand in a meaningful way. But in order to be truly effective, sponsorships must be aligned with a company’s brand values. For pharmaceutical companies, this means aligning sponsorships with causes that are related to health and wellness.

Pharmaceutical Technology
JULY 19, 2023
Flagship Pioneering has entered into a collaboration with Pfizer to develop a new innovative therapy pipeline.

XTalks
JULY 19, 2023
As the pressure builds on Kaiser Permanente, an 85,000-member strong unionized healthcare workforce is upping the ante in the negotiation of a new labor contract. The Coalition of Kaiser Permanente Unions announced this week that its members will stage pickets at 50 Kaiser Permanente facilities spread across California, Washington, Oregon and Colorado, sparking fears of a Kaiser Permanente strike.

Pharma Marketing Network
JULY 19, 2023
Sponsorships can be a powerful tool for pharmaceutical companies to engage with their target audience, build relationships with key stakeholders, and promote their brand in a meaningful way. But with so many different healthcare organizations and causes to choose from, it can be difficult to know where to start. Here are some tips for choosing the right sponsorships for maximum impact in the pharmaceutical industry: Start with your goals: What do you hope to achieve through your sponsorship?

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Fierce Pharma
JULY 19, 2023
After Novo Nordisk's popular obesity medicine Wegovy went into short supply, people turned to the company first-generation weight-loss drug, Saxenda, for treatment. | After Novo Nordisk's Wegovy became hard to come by, people turned to the company first-generation weight loss drug, Saxenda, for treatment. Now, the company is struggling to handle demand for that product.

Pharma Marketing Network
JULY 19, 2023
The pharmaceutical industry is constantly evolving, and digital technology is playing an increasingly important role in marketing campaigns. In the past, pharmaceutical companies relied heavily on traditional marketing methods such as print ads, TV commercials, and direct mail. However, these methods are becoming less effective as consumers are increasingly turning to digital channels for information and research.

BioPharma Reporter
JULY 19, 2023
Liz Beatty is co-founder and chief strategy officer at Inato, a clinical trials platform which flips the traditional model and allows community sites to participate in the trials that are best aligned with their interests and that of their patients.

Pharma Times
JULY 19, 2023
Illumina and Nashville Biosciences’ have announced AbbVie, Amgen, AstraZeneca, Bayer and Merck as founding members - News - PharmaTimes

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Fierce Pharma
JULY 19, 2023
While vaccines for COVID-19 have certainly enjoyed the spotlight in recent years, rates of uptake for other shots have sharply declined. | The GSK-funded report looked at adult vaccination data and found a major slump in vaccine adoption over the last few years, save for COVID vaccines.

Pharma Times
JULY 19, 2023
By combining both netarsudil and latanoprost treatment, Roclanda reduces intraocular pressure - News - PharmaTimes

Fierce Pharma
JULY 19, 2023
Right on the heels of a major study win for its star drug Vyvgart, argenx has raised more than $1 billion in cash to support its operations. | Right on the heels of a major study win for its star drug Vyvgart, argenx has raised more than $1 billion in cash to support its operations.
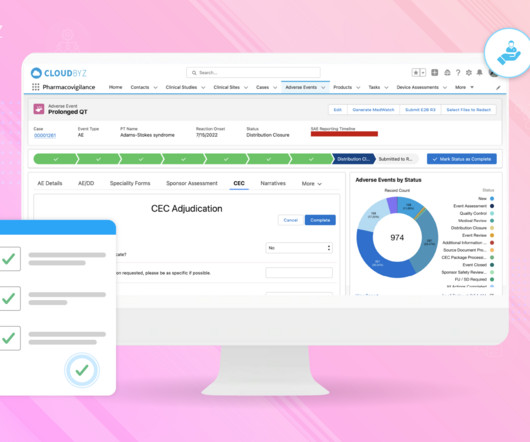
Cloudbyz
JULY 19, 2023
Clinical trials are vital in advancing medical knowledge and developing new treatments. However, ensuring participant safety is paramount throughout the trial process. Pharmacovigilance plays a critical role in monitoring adverse events, collecting safety data, and reporting requirements to protect participants. This blog delves into the significance of pharmacovigilance in clinical trials, highlighting its role in participant safety, data integrity, and regulatory compliance.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content