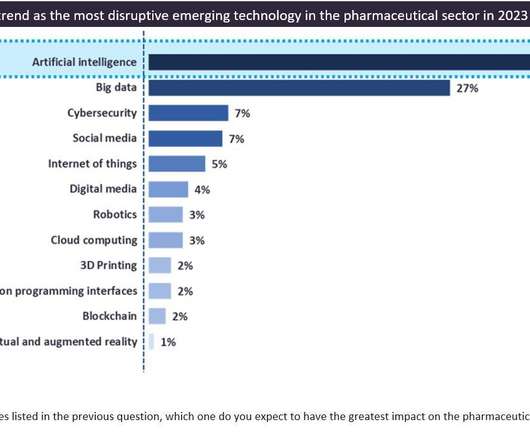
AI continues to gain momentum in the biopharmaceutical industry in 2023
Pharmaceutical Technology
JANUARY 16, 2023
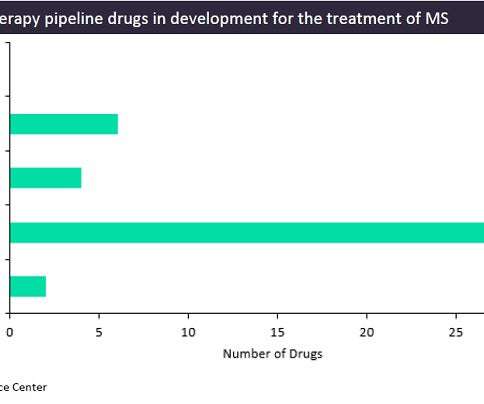
While the biopharmaceutical industry has been impacted and pressured by various factors such as the Covid-19 pandemic, inflation, the Ukraine-Russia war, ongoing supply chain issues, and a challenging economic environment, collaboration between pharma companies and emerging technologies providers continues to grow, especially in the research and development (R&D) field, offering some resilience in times of geopolitical and economic disruptions.


































Let's personalize your content