Avacta doses first patient in pivotal phase 1a trial
Pharma Times
APRIL 5, 2023
Individual receives AVA6000 treatment as part of additional dose escalating study

Pharma Times
APRIL 5, 2023
Individual receives AVA6000 treatment as part of additional dose escalating study

Bio Pharma Dive
APRIL 3, 2023
By the end of June, the agency could clear a gene therapy for Duchenne muscular dystrophy and two vaccines for RSV, as well as issue a precedent-setting decision on a closely watched ALS drug.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
APRIL 3, 2023
The World Health Organisation (WHO) has revised its recommendations regarding the use of Covid-19 vaccines following a meeting of the agency’s Strategic Advisory Group of Experts on Immunisation (SAGE). The latest guidance applies to the current phase of the pandemic and reflects the impact of the Omicron variant, which has led to high levels of immunity in all age groups through both vaccination efforts and infections across the globe.
NPR Health - Shots
APRIL 7, 2023
A federal judge in Texas stayed the FDA's approval of the drug mifepristone, while a federal judge in Washington state blocked any FDA change in access. (Image credit: Allen G.

Medical Xpress
APRIL 6, 2023
Broccoli is known to be beneficial to our health. For example, research has shown that increased consumption of the cruciferous vegetable decreases incidences of cancer and type 2 diabetes. In a recent study, researchers at Penn State found that broccoli contains certain molecules that bind to a receptor within mice and help to protect the lining of the small intestine, thereby inhibiting the development of disease.

Bio Pharma Dive
APRIL 2, 2023
The startup, a successor to an eye gene therapy biotech that Novartis bought in 2020, will wind down after preclinical experiments didn’t meet the bar set by its leaders.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
NPR Health - Shots
APRIL 6, 2023
When she gave birth to her baby with a fatal condition two months early, Samantha Casiano scrambled to raise funds for the funeral. Anti-abortion advocates say Texas laws are "working as designed.
Medical Xpress
APRIL 4, 2023
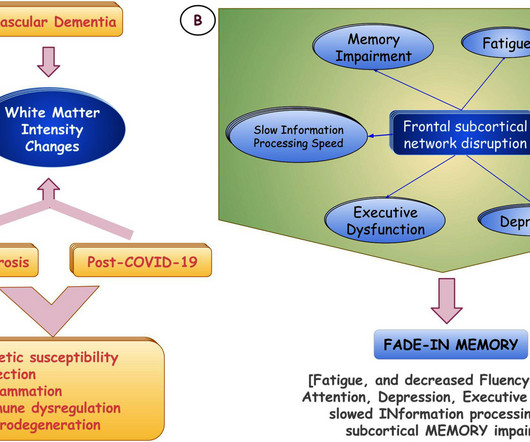
Infection with SARS-CoV-2 has a significant impact on cognitive function in patients with preexisting dementia, according to new research published in the Journal of Alzheimer's Disease Reports. Patients with all subtypes of dementia included in the study experienced rapidly progressive dementia following infection with SARS-CoV-2.

Bio Pharma Dive
APRIL 4, 2023
The first-line approval of Padcev together with Keytruda is viewed as important to expanding the drug’s market, and comes weeks after Pfizer agreed to buy Seagen for $43 billion.

Pharmaceutical Technology
APRIL 6, 2023
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has granted ADvantage Therapeutics’ immunotherapy AD04 an Innovation Passport for the treatment of Alzheimer’s disease. The designation, under the regulator’s Innovative Licensing and Access Pathway (ILAP), will fast-track a potential route to market for AD04 by providing collaborative opportunities with UK institutes like the National Institute for Health and Care Excellence (NICE).

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

STAT News
APRIL 3, 2023
Every hospital in America promises to protect the privacy of its patients and the details of their medical care. And almost every one of them uses sophisticated data tools to track and share the personal information of visitors as soon as they start clicking on their websites. A new study found that 99% of U.S. hospitals employed online data trackers in 2021 that transmitted visitors’ information to a broad network of outside parties, including major technology companies, data brokers, an

BioPharma Reporter
APRIL 6, 2023
Meissner Corporation â a company which manufactures advanced microfiltration and therapeutic manufacturing systems for pharmaceutical drugs, therapeutics, biologics, and cell and gene therapies - will invest nearly $250m in a new manufacturing facility in Athens-Clarke County in the US.

Bio Pharma Dive
APRIL 7, 2023
In paring back use of Imbruvica, the drugmaker has become the latest developer to voluntarily withdraw indications for a cancer medicine following a setback in confirmatory testing.
Pharmaceutical Technology
APRIL 3, 2023
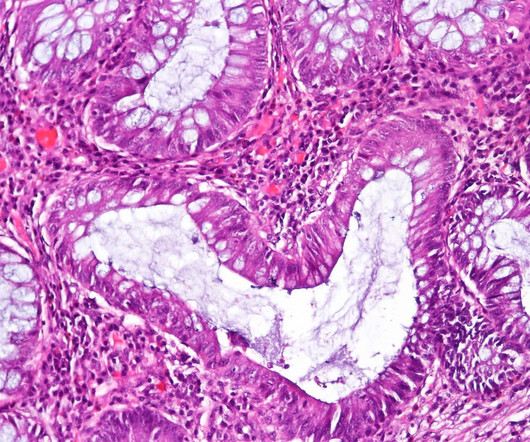
The European Commission (EC) has granted marketing authorisation for Sandoz’s biosimilar Hyrimoz (adalimumab) citrate-free high-concentration formulation (HCF). Hyrimoz has been approved for use in all the indications covered by the reference medicine Humira, including plaque psoriasis, rheumatic diseases, ulcerative colitis, Crohn’s disease, uveitis and hidradenitis suppurativa.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

STAT News
APRIL 7, 2023
In a case that could reshape pregnancy in America, a federal judge on Friday sided with anti-abortion groups seeking a nationwide ban on abortion pills, ruling that the Food and Drug Administration acted improperly in approving mifepristone in 2000. The ruling will go into effect in seven days if a stay is not granted by an appeals court or by the Supreme Court.
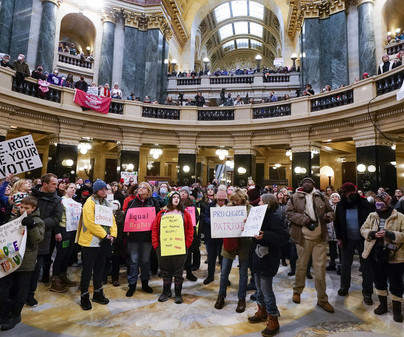
NPR Health - Shots
APRIL 4, 2023
It's the final day to vote in Wisconsin's Supreme Court race that's broken national spending records. The winner could be the swing vote on issues like abortion, redistricting and election lawsuits.
Bio Pharma Dive
APRIL 4, 2023
The U.K. biotech will lay off 30% of its workforce and focus on an experimental Gaucher disease treatment, five months after an initial restructuring that cut a hemophilia gene therapy.
Pharmaceutical Technology
APRIL 4, 2023
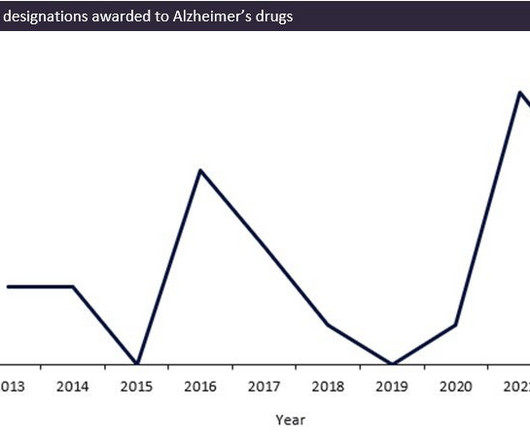
The FDA has seen a record surge in review designations being awarded over the last two years for Alzheimer’s indications, with 12 review designations being awarded to drugs between 2020 and 2022. This coincided with the much-anticipated wave of monoclonal antibody drugs for Alzheimer’s disease (AD) such as Eisai/Biogen’s Leqembi (lecanemab-irmb) and Eli Lilly’s donanemab, which are predicted to provide significant improvement on previous AD therapies.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

STAT News
APRIL 4, 2023
For the first time, Mark Cuban’s Cost Plus Drug Company is selling medicines made by a large drug manufacturer directly to consumers at a greatly reduced price, the latest sign that the billionaire is trying to make good on his vow to disrupt the opaque pharmaceutical supply chain. Until now, the company has focused on selling generic versions of brand-name medicines.

NPR Health - Shots
APRIL 3, 2023
Art can make the brain's wiring stronger, more flexible, and ready to learn, say the authors of the new book, Your Brain on Art: How the Arts Transform Us.
Bio Pharma Dive
APRIL 5, 2023
Results from two large clinical trials, published in the high-profile medical journal Wednesday, detail the shot’s safety and efficacy against the common respiratory infection.

Pharmaceutical Technology
APRIL 3, 2023
Sartorius , through its French listed sub-group Sartorius Stedim Biotech , has signed an agreement to acquire Polyplus for €2.4bn ($2.6bn). The deal will see Polyplus join the German life science group’s portfolio allowing the latter to leverage expertise in transfection reagents and plasmid DNA for gene therapy. Polyplus, based in Strasbourg, France, produces key components in the production of viral vectors used in cell and gene therapies.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
STAT News
APRIL 3, 2023
One in six people across the globe face infertility at some point in their lifetime, according to the first new estimates from the World Health Organization in a decade. The prevalence is “staggering,” Pascale Allotey, director of sexual and reproductive health and research at WHO, said at a press conference Monday. The report estimates that 17.8% of adults in high-income countries and 16.5% in low- and middle-income countries experience infertility.

NPR Health - Shots
APRIL 2, 2023
Patients who have digestive symptoms only after eating red meat may have developed an allergy caused by ticks. Previously, doctors looked for symptoms such as rashes, hives and breathing troubles.
Bio Pharma Dive
APRIL 3, 2023
The approval application is the first in the U.S. for a CRISPR-based medicine and puts the partners ahead of a rival therapy from Bluebird bio.
Pharmaceutical Technology
APRIL 3, 2023
BioNTech has signed exclusive licence and collaboration agreements with Duality Biologics (DualityBio) for the development of two antibody-drug conjugate (ADC) assets for solid tumours. The agreements also include the manufacturing and commercialisation of the two assets, including DB-1303 and DB-1311, across the globe. As part of the new deals, BioNTech will have commercial rights to the ADCs worldwide, excluding mainland China, the Hong Kong special administrative region and the Macau special

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

STAT News
APRIL 3, 2023
Here is STAT’s biotech scorecard, our regular ledger of stock-moving biotech events, for the second quarter: When is “mid-year”? This is not an existential question. Practically speaking, when a drugmaker, let’s say Bridge Bio , tells investors that an important readout of all-cause mortality data from a clinical trial of its heart drug acoramidis is coming “mid-2023,” does that mean the end of June or the beginning of July?

NPR Health - Shots
APRIL 3, 2023
The Be My Eyes app pairs those with visual impairments with human volunteers. It's a form of micro-volunteering that has brought people together. Now, AI is changing it.

Bio Pharma Dive
APRIL 4, 2023
Hiring Adam Keeney, a Sanofi veteran, to lead corporate development could be seen as timely, given that dealmaking has come into focus at Biogen since the recent appointment of Chris Viehbacher as CEO.

Pharmaceutical Technology
APRIL 5, 2023
Austrian molecular glue degraders discovery and development company Proxygen and Merck , known as MSD outside the US and Canada, have entered into a multi-year research collaboration and licence deal. Through the collaboration, the companies will work together to identify and develop molecular glue degraders against multiple therapeutic targets. MSD will make an upfront payment to Proxygen, which will also be eligible for up to $2.55bn in future payments, subject to the achievement of agreed res

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
Let's personalize your content