US FDA grants orphan drug status to IN8bio’s INB-400 and INB-410
Pharmaceutical Technology
APRIL 26, 2023
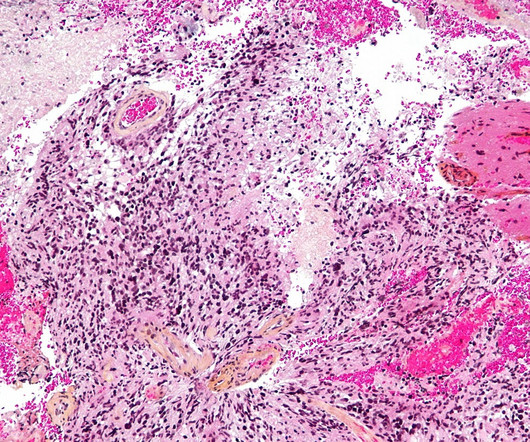
This marks the first-ever designation for genetically modified gamma-delta T cell therapies. INB-400, an autologous, genetically engineered gamma-delta T cell therapy, is the company’s DeltEx chemotherapy-resistant autologous and allogeneic drug-resistant immunotherapy (DRI) technology.






















Let's personalize your content