Rezvilutamide by Jiangsu Hengrui Medicine for Prostate Cancer: Likelihood of Approval
Pharmaceutical Technology
JUNE 5, 2023
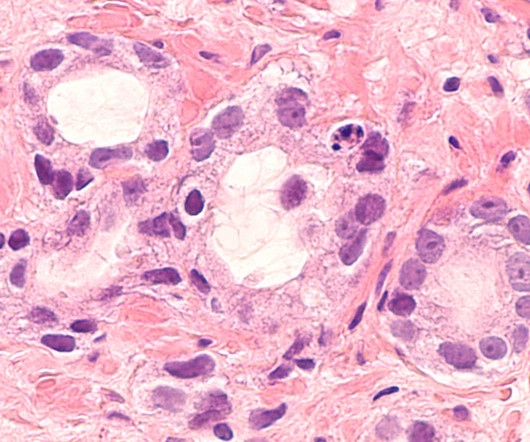
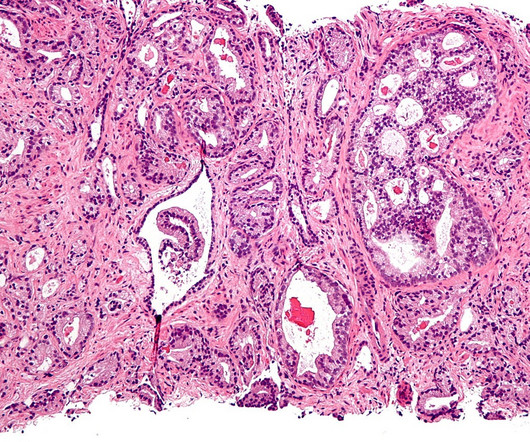
Rezvilutamide is under clinical development by Jiangsu Hengrui Medicine and currently in Phase III for Prostate Cancer. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. Rezvilutamide overview Rezvilutamide is an antineoplastic agent.




































Let's personalize your content