Interview with Cody Simmons, CEO of DermaSensor, Maker of Handheld Skin Cancer Evaluation Tool – Xtalks Life Science Podcast Ep. 107
XTalks
APRIL 26, 2023
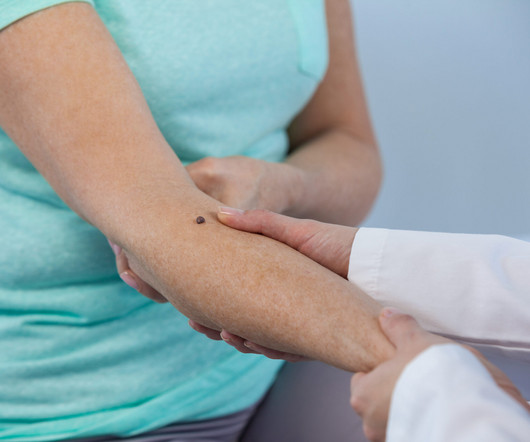
Hear more about the innovative technology and application of DermaSensor in this episode. Carli P, Nardini P, Crocetti E, De Giorgi V, Giannotti B. Stanganelli I, Serafini M, Bucch L. Seiverling EV, Agresta T, Cyr P, Caines L, Nguyen VL, Chatha K, Siegel DM. Melanoma Res 2004;14(5):403-407. Dermatology.








































Let's personalize your content