Modernizing cell culture processes for the next wave of genomic medicine
Pharmaceutical Technology
FEBRUARY 3, 2023
With scientists fervently developing mRNA vaccines, nucleic acid therapeutics, and viral vector-based gene therapies, clinicians are set to have a growing number of tools available to treat a wide range of conditions, from infectious diseases to genetic disorders and more. The field of genomic medicine has reached a true turning point.


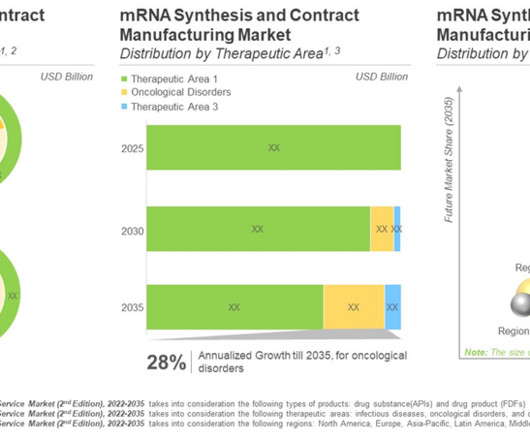
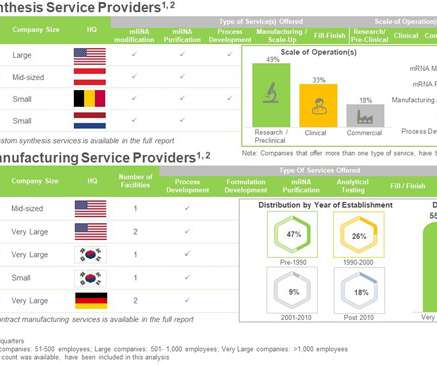





















Let's personalize your content