How Does Site Location Impact Patient Diversity in Clinical Trials?
Velocity Clinical Research
APRIL 5, 2024
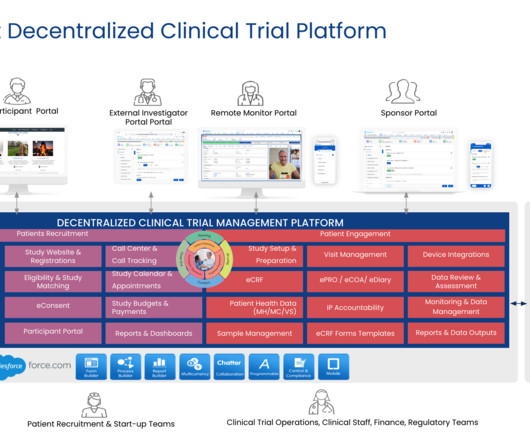
Velocity recently asked some key employees to come together and discuss a wide array of issues impacting diversity, equity, and inclusion (DEI) in clinical trials. It’s an issue Velocity Clinical Research takes very seriously from the earliest stages of site location planning, says Brandi Lang , Chief Legal Officer for Velocity.


















































Let's personalize your content