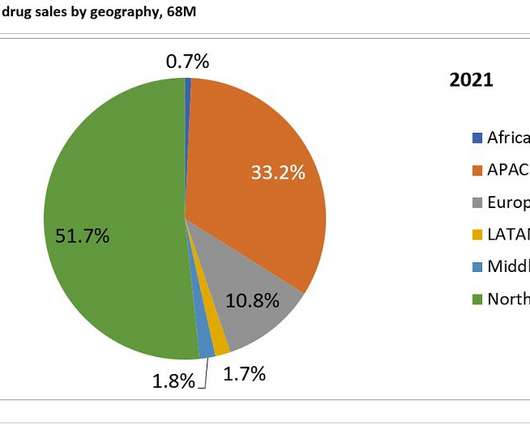
Biogen’s Alzheimer’s drug: What’s the price tag on hope?
World of DTC Marketing
JUNE 1, 2021
After the CEO departed the sinking ship, new management pinned all their hopes on the Biogen drug for Alzheimer’s. The first clinic; trials were everything but encouraging but so much depend ended on this drug’s approval that Biogen returned and recited the data. Too many doctors, the data was still questionable.

















































Let's personalize your content