Cannabinoids receptors: popular preclinical target but banned in 137 countries
Pharmaceutical Technology
DECEMBER 5, 2022
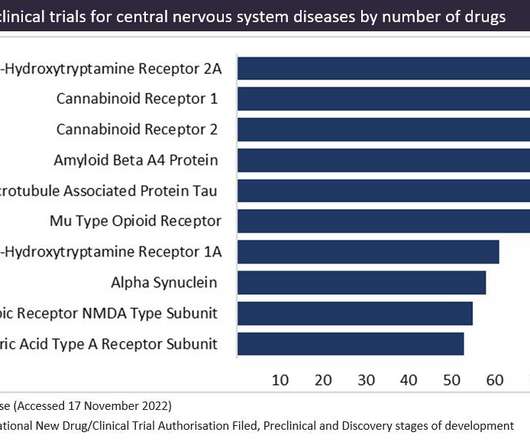
CB1 receptors are primarily expressed in the brain and central nervous system, whereas CB2 receptors are expressed in T-cells in the immune system. Figure 1 shows the targets in preclinical trials for central nervous system diseases, as sourced from GlobalData’s Drugs Database.































Let's personalize your content