NorthSea Therapeutics bags FDA rare paediatric disease tag for NASH drug
Pharmaceutical Technology
OCTOBER 17, 2023
The Netherlands-based company’s treatment has been awarded the designation by the US FDA following a successful Phase I trial.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 tag rare-diseases
tag rare-diseases 
Pharmaceutical Technology
OCTOBER 17, 2023
The Netherlands-based company’s treatment has been awarded the designation by the US FDA following a successful Phase I trial.

pharmaphorum
MARCH 20, 2024
Orchard Therapeutics has revealed the US price of Lenmeldy, its gene therapy for rare disease MLD, placing a $4.25m price tag on the one-shot treatment
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
SEPTEMBER 30, 2022
Skysona is indicated as a one-time gene therapy to slow the progression of cerebral adrenoleukodystrophy (CALD), a rare paediatric neurodegenerative disease in boys aged 4–17 years diagnosed with early-stage CALD. Prior to bluebird's approvals, there were only two FDA-approved gene therapies for inherited conditions on the market.

Pharmaceutical Commerce
MARCH 6, 2024
Navigating the complex landscape of healthcare coverage can be an intimidating task, especially for patients with rare diseases for which treatments often come with a high price tag.
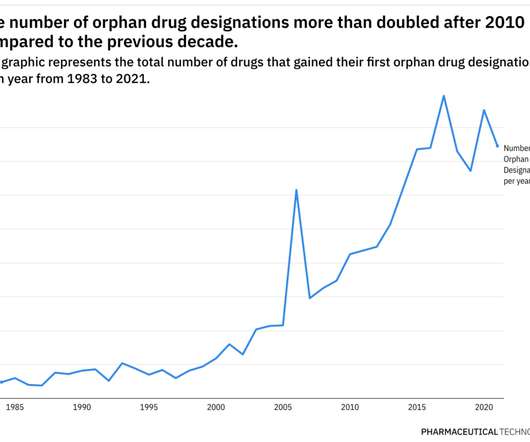
Pharmaceutical Technology
JUNE 29, 2022
This year has already been eventful when it comes to the development of therapies for rare diseases. Additionally, pricing and access for rare disease therapies continue to be scrutinized closely. Prior to the program, only 10 drugs were approved for a rare disease.
XTalks
FEBRUARY 27, 2024
Rare Disease Day 2024, which falls on February 29 this year, is an opportunity to unite under a common cause: to bring attention to the challenges faced by those living with rare diseases and to push for advancements in research, treatment and policy.

Rethinking Clinical Trials
JANUARY 23, 2024
People with angina-like symptoms are often not patients with a disease. Most do not have obstructive coronary artery disease (CAD), but a few are very high risk. Costs are rarely a significant factor in comparing different testing approaches. They are trying to follow the guidelines for symptoms and disease.
STAT News
OCTOBER 6, 2022
… Multimillion-dollar prices for a rival’s treatments with the potential to cure rare diseases are “in the right ballpark,” according to Stuart Arbuckle, chief operating officer at Vertex Pharmaceuticals, which is preparing to market its own treatment for sickle-cell disease , Bloomberg News tells us. Even
Pharmaceutical Technology
JULY 14, 2022
It is estimated that there are currently more than 7,000 orphan diseases, many of which are considered life-threatening and most of which have a genetic basis. Despite this high number, orphan diseases are rare by definition, affecting around one in 2,000 people as defined by the European Union. Go-to-market strategies.
Pharmaceutical Technology
FEBRUARY 10, 2023
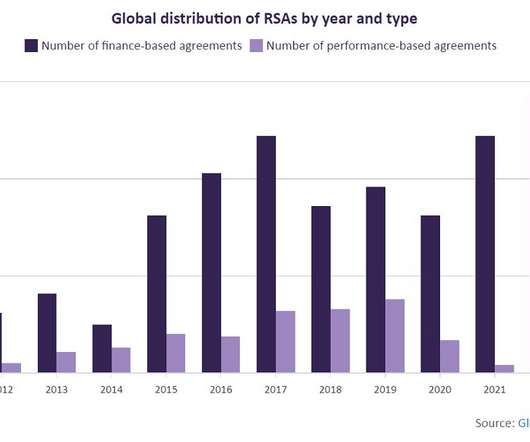
This is not the first treatment to come with a high price tag. However, more highly innovative/high-cost treatments, within infectious disease and neurology space, are likely to enter the market for rare disorders, increasing the number of non-oncology RSAs.

XTalks
AUGUST 19, 2022
But bluebird bio has said such setbacks are expected for a company at the frontier of developing gene therapies for rare diseases. The most severe form of the disease is sometimes called transfusion-dependent beta-thalassemia or beta-thalassemia major. Zynteglo’s hefty price tag of $2.8

XTalks
JUNE 28, 2023
DMD is a rare genetic disorder that leads to progressive muscle degeneration and weakness. In its approval, the FDA said it considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease for these children and the urgent unmet medical need.

XTalks
DECEMBER 13, 2023
The US Food and Drug Administration (FDA) has approved the first gene therapies for the treatment of sickle cell disease, approving two on the same day. Both gene therapies are approved for individuals 12 years of age and older with sickle cell disease. Casgevy is also the first ever CRISPR/Cas9-based therapy approved in the US.
pharmaphorum
JUNE 2, 2022
R&D into orphan drugs is growing alongside the number of approved treatments, providing treatments for rare diseases that previously did not have any. As covered in a previous article , the importance of R&D to develop treatments for rare diseases is high. of the global population are affected by a rare disease.

XTalks
NOVEMBER 24, 2022
Prior to Hemgenix, bluebird bio’s recently approved gene therapy Skysona for the rare neurological disorder cerebral adrenoleukodystrophy (CALD) held the record for the highest-costing drug in the world at $3 million and before that, it was Novartis’ one-dose gene therapy Zolgensma which is priced at about $2 million. With a list price of $3.5

pharmaphorum
NOVEMBER 19, 2021
Roche’s oral treatment Evrysdi for the rare genetic disease spinal muscular atrophy (SMA) will be made available on the NHS in England, after NICE reached a three-year access agreement with the company. million price tag make it is the most expensive treatment ever approved for NHS funding.

XTalks
JANUARY 28, 2022
Uveal melanoma is a rare and aggressive type of melanoma of the eye. Up to 50 percent of patients will develop metastatic disease, mainly spreading to the liver, making treatment difficult and limited. The new treatment will thus help address the unmet need for the disease. The median treatment time is about 23 weeks (or 5.3

XTalks
SEPTEMBER 27, 2022
Skysona received FDA approval for the treatment of the rare neurological disorder cerebral adrenoleukodystrophy (CALD). CALD is a rare, progressive neurodegenerative disease that mainly affects young boys. The record-breaking price tag of Skysona is not surprising for one-time gene therapies like it.
pharmaphorum
OCTOBER 21, 2021
Vounatsos also insisted that Aduhelm’s price tag has not been a factor discouraging treatment with the drug. Biogen stumped up $1.525 billion upfront for rights to the drug and another Sage candidate for neurological diseases last year, when the expectation was that Aduhelm was unlikely to get FDA approval.
XTalks
OCTOBER 26, 2021
After initial rejection from the National Institute for Health and Care Excellence (NICE) last year, the non-departmental public body of the Department of Health in England has now given the green light to the gene silencing treatment Givlaari (givosiran) for the treatment of the rare metabolic disorder, acute intermittent porphyria (AIP).

pharmaphorum
APRIL 5, 2022
Uveal melanoma is a rare cancer of the eye, which in the majority of patients is localised. mUM is the most common form of cancer affecting the eye in adults, but is still rare, diagnosed in a around 1,700 patients each year in the US, with a few hundred already with metastatic disease that has spread to other tissues.

pharmaphorum
JANUARY 11, 2021
Under the plans the company’s rare disease drugs will remain under the aegis of bluebird with current genetic disease president Andrew Obenshain taking the reins as CEO. million price tag. Zynteglo is already in beta-thalassemia in Europe, where the company will seek to expand access despite its hefty $1.8

pharmaphorum
DECEMBER 12, 2022
Dutch biotech Argenx is on course to add another rare disease indication to the label of its FcRn blocker Vyvgart – primary immune thrombocytopenia (ITP) – thanks to new data reported at the ASH annual meeting.

XTalks
JUNE 8, 2021
In addition, recent data from a Phase I trial has shown ‘remarkable’ long-term results of the gene therapy in children with the disease. With a price tag of over $2.5 While SMN1 gene mutations cause the disease, the number of copies of the SMN2 gene modifies the severity and helps determine the type of the condition.

Intouch Solutions
JULY 27, 2021
they pointed out that the COVID-19 vaccines, and the controversial approval of aducanumab for the treatment of Alzheimer’s disease, represented polar opposites in terms of drug pricing reform. In their letter, “ Drug Pricing Reform in 2021 – Going Big or Going Bipartisan? In the case of the vaccines, the U.S.

XTalks
DECEMBER 21, 2022
Bluebird is also now back on track in evaluating its candidate gene therapy for sickle cell disease (SCD) after the FDA lifted a clinical hold on studies of the drug this month. There is also generally a low cost of return for gene therapies, particularly those for rare diseases. million for a single infusion.

pharmaphorum
MAY 17, 2021
C5 inhibitor Soliris – which has a list price in the US of more than $500,000 per year – has been a standard therapy for the rare disorder since 2007, and was joined on the market by longer-acting follow-up Ultomiris in 2018 which launched with a price tag of around $450,000 per year. billion and $1.1 billion and $1.1

pharmaphorum
JUNE 2, 2021
The cost-effectiveness agency’s initial assessment is that Evrysdi is simply too expensive at its current price to be provided to the roughly 1,500 people with the rare genetic disorder who might be eligible to receive it. Zolgensma was backed for type 1 SMA in draft guidance issued last month, even though the gene therapy’s £1.79
XTalks
NOVEMBER 27, 2021
The technical advisory group on SARS-CoV-2 virus evolution (TAG-VE) met on Friday to discuss B.1.1.529, Scientists in South Africa first detected the lineage a week ago upon which the National Institute for Communicable Diseases of the country alerted global health authorities.

Intouch Solutions
APRIL 11, 2024
This can make a difference in all cases, but particularly with rare diseases. Offering the physician support from a virtual assistant during the differential diagnosis process where they have access to a more extensive knowledge and experience base.
XTalks
MARCH 22, 2023
The years that followed have seen a rise in large pharma companies acquiring much smaller biopharmas with a narrow focus on rare disease therapeutic development. The proton-pump inhibitor is designed to reduce stomach acid levels, thereby treating digestive conditions like gastroesophageal reflux disease (GERD).
The Pharma Data
OCTOBER 21, 2020
21, 2020 — A combination of two “targeted” therapies can beat back a rare form of blood cancer — without the toxic effects of chemotherapy, a new study has found. The disease is a subtype of ALL in which the cancer cells have a genetic abnormality called the Philadelphia chromosome (Ph). WEDNESDAY, Oct.
The Pharma Data
OCTOBER 21, 2020
21, 2020 — A combination of two “targeted” therapies can beat back a rare form of blood cancer — without the toxic effects of chemotherapy, a new study has found. The disease is a subtype of ALL in which the cancer cells have a genetic abnormality called the Philadelphia chromosome (Ph). WEDNESDAY, Oct.

Pharmaceutical Technology
JANUARY 27, 2023
On January 18, Sanofi launched a new warranty program for its rare blood disorder drug Cablivi (caplacizumab), which tackles value-based agreements in a slightly different way. A refund up to 12 inpatient doses will also be offered for patients whose disease worsens while receiving treatment.

FDA Law Blog
JUNE 27, 2023
Amongst his accomplishments, Law360 considered the role James has played in leveraging little-used pathways to FDA approval for often first-ever drugs to treat rare diseases (e.g., Duchenne, Friederichs’s Ataxia, Sickle Cell Disease, Chagas, ALS). Dormer Jeffrey N. Gibbs Paul M. Hyman Alan M. Kirschenbaum Allyson B.

pharmaphorum
AUGUST 26, 2022
BioMarin will no doubt have an eye on the earlier experience of bluebird bio, which secured EU approval for rare disease gene therapy Zynteglo (betibeglogene autotemcel) in 2019 for beta thalassaemia with a price tag of around €1.6 million spread over five years.

pharmaphorum
AUGUST 10, 2020
Evrysdi is the third treatment approved for SMA , an ultra-rare muscle wasting disease that can begin in early childhood, after Biogen’s Spinraza (nusinersen) and Novartis’ Zolgensma (onasemnogene abeparvovec). But Spinraza costs $750,000 in the first year of treatment and about half that price annually from then on.

The Pharma Data
MAY 4, 2021
Rare Disease. In March 2021, Pfizer, the Israel Ministry of Health (MoH) and BioNTech announced real-world evidence demonstrating dramatically lower incidence rates of COVID-19 disease in individuals fully vaccinated with BNT162b2. The vaccine was 100% effective against severe disease as defined by the U.S. Total Revenue.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content