Cartherics grants licence for CTH-004 to Shunxi
Pharmaceutical Technology
MAY 1, 2023
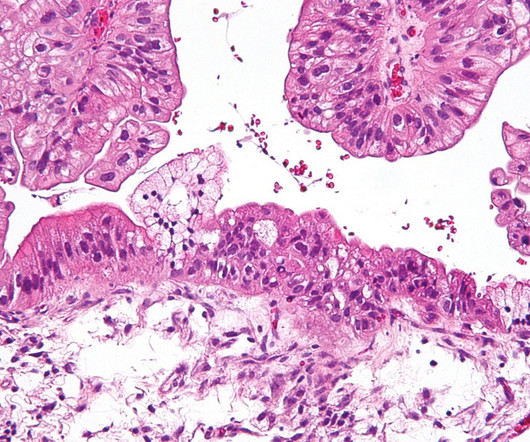
Australian biotechnology company Cartherics has granted licence for CTH-004 , its autologous CAR-T cell product, to Chinese company Shunxi. Shunxi will also hold an option to negotiate rights to other CAR-T products, which include the licenced IP. In animal models of ovarian cancer, it demonstrated promising results.









































Let's personalize your content