The Importance of Hazard Communications in Clinical Trials Involving Genetic Engineering
Advarra
JUNE 13, 2024
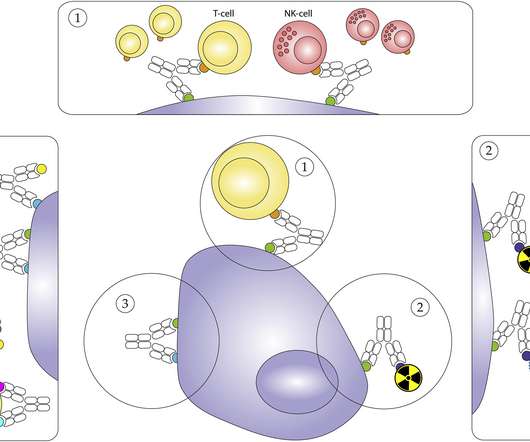
Recombinant DNA technologies and genetically modified biological agents are being adapted for a wide scope of therapeutic applications, and their use is becoming increasingly common in clinical trials. Clinical personnel need to understand these risks and how risks are differentiated between healthy and vulnerable individuals.





































Let's personalize your content