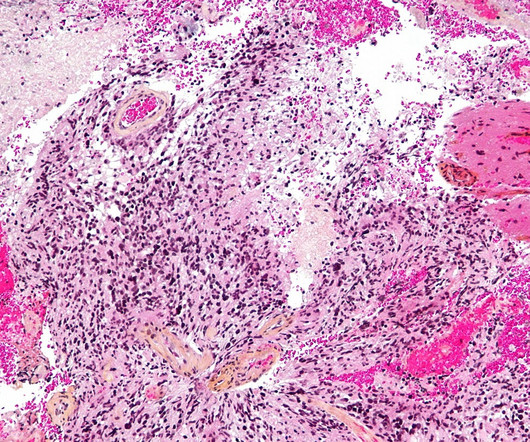
CIGB-128 by Center for Genetic Engineering and Biotechnology for Brain Tumor: Likelihood of Approval
Pharmaceutical Technology
JUNE 10, 2023
CIGB-128 is under clinical development by Center for Genetic Engineering and Biotechnology and currently in Phase I for Brain Tumor. According to GlobalData, Phase I drugs for Brain Tumor does not have sufficient historical data to build an indication benchmark PTSR for Phase I. Buy the report here.



































Let's personalize your content