The Significance of the MHRA Approval and Upcoming FDA Review of the First Gene Editing Treatment
Worldwide Clinical Trials
NOVEMBER 27, 2023
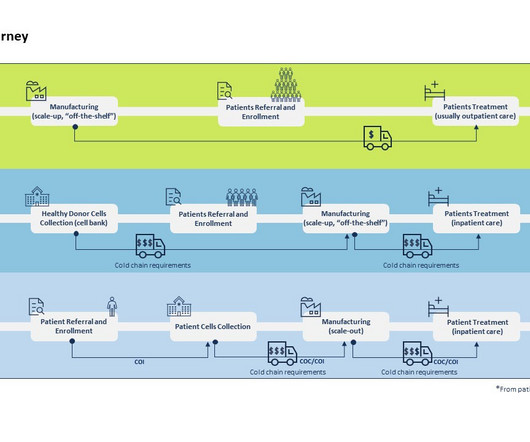
Casgevy, the commercial product formerly known as exa-cel, is administered by taking stem cells out of a patient’s bone marrow and editing a gene in the cells in a laboratory, with the modified cells then infused back into the patient after conditioning treatment to prepare the bone marrow. In June 2023, the U.S.









































Let's personalize your content