How using AI in clinical trials accelerates drug development
Pharmaceutical Technology
JUNE 15, 2023
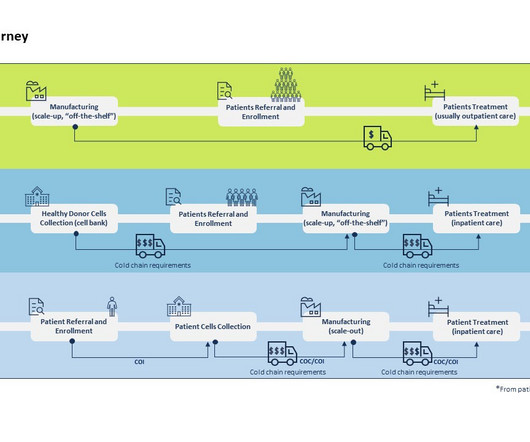
But before pharmaceutical companies can go to market with a breakthrough drug, they need to ensure safety and efficacy through clinical trials. Pharma R&D teams are solving this problem by leveraging the power of artificial intelligence (AI) in clinical trials to save time and money.








































Let's personalize your content