Flamingo gets grant from VLAIO to accelerate RNA-focused oncology portfolio
Pharmaceutical Technology
JUNE 16, 2023
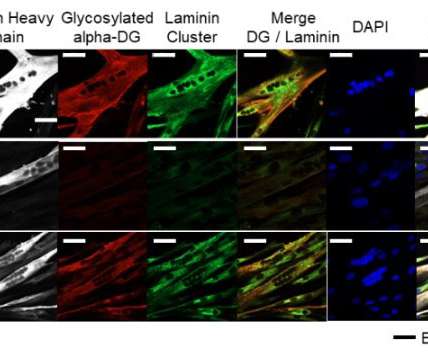
from Flanders Innovation & Entrepreneurship (VLAIO) to further advance its oncology portfolio targeting RNA. The grant will help Flamingo to support its translational research in a Phase II study of its lead clinical programme, danvatirsen, to treat head and neck squamous cell carcinoma.


































Let's personalize your content