Sarepta’s Elevidys Reaches Finish Line as First Gene Therapy Approved for Duchenne Muscular Dystrophy
XTalks
JUNE 28, 2023
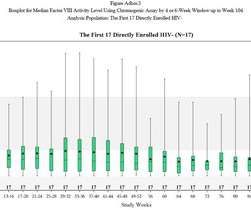
Gene therapies for Duchenne muscular dystrophy (DMD) have been an area of intense research and Sarepta’s Elevidys is now the first one to be approved by the US Food and Drug Administration (FDA). DMD is caused by the absence of dystrophin, a protein that helps maintain the integrity of muscle cells.










































Let's personalize your content