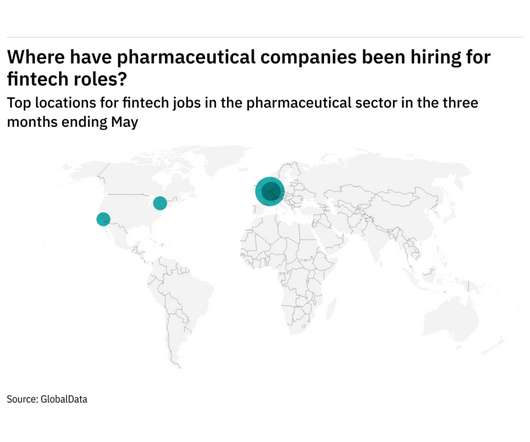
What to know about polio, a disease once again vying for attention
STAT News
JULY 26, 2022
Depending on how young you are, your mother may remember polio. Your grandmother, though, would likely shudder at the mere mention of the word, which for years evoked images of iron lungs, of paralyzed children, of summers when fevers struck fear in parents’ hearts. But if you are in your 20s, 30s, 40s, or even 50s, it’s possible you don’t know much at all about the disease caused by a trio of viruses that have long been vanquished from North America and most of the develope








































Let's personalize your content