How a busy year for biotech IPOs measures up at the three-quarter mark
Bio Pharma Dive
OCTOBER 4, 2021
Drugmaker initial public offerings could break records set just a year ago. But valuations and stock performance have slipped from historic highs.

Bio Pharma Dive
OCTOBER 4, 2021
Drugmaker initial public offerings could break records set just a year ago. But valuations and stock performance have slipped from historic highs.

Pharma Mirror
OCTOBER 4, 2021
By Kambiz Shekdar, Ph.D. Open source drug discovery was proposed in the past in connection with third-world diseases like tuberculosis and malaria, but it is in the context of first-world indications where it is needed most. Despite untold investment by numerous pharmaceutical companies, FDA-approved drugs that target critical brain functions and conditions like anxiety, depression, and sedation continue to present severe and unpredictable side effects, including suicidal ideation.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
OCTOBER 4, 2021
Initiation of the Phase 3 trial is an important milestone for the biotech after earlier setbacks, as well as for patients with the inherited muscle disease.

World of DTC Marketing
OCTOBER 4, 2021
SUMMARY: The Facebook whistleblower lawsuit can be summed up in one sentence: Facebook prioritizes engagement above all else because it leads to more profits even if that engagement containing inflammatory information. Earlier this year, I was asked by a client to write a position paper on using Facebook for the DTC marketing of an oncology product.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Bio Pharma Dive
OCTOBER 4, 2021
Tecartus, Gilead's second cell therapy on the market, is the first CAR-T treatment to be cleared for use in people older than 18 with ALL.
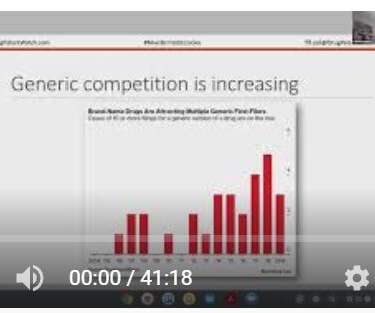
Drug Patent Watch
OCTOBER 4, 2021
This is my talk from the 14th Annual Portfolio Planning and Partnership for Generic Conference. I discuss: Identifying generic entry opportunities at the earliest stages Discovering opportunities that avoid litigation…. The post Generic Portfolio Management, Partnering with Brands, and Staying Competitive appeared first on DrugPatentWatch - Make Better Decisions.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

BioSpace
OCTOBER 4, 2021
After serving three presidential administrations, Dr. Francis S. Collins is stepping down as director of the National Institutes of Health at the end of the year.

Pharma Times
OCTOBER 4, 2021
MSD and Ridgeback Therapeutics announced that their investigational anti-viral medicine reduced the risk of hospitalisation and death by approximately 50% in non-hospitalised adult patients with mild-to-moderate COVID-19.

pharmaphorum
OCTOBER 4, 2021
NHSX, the government agency tasked with digitally transforming NHS England, has granted funding to 14 projects in its first digital health award competition. The Digital Health Partnership Award opened for applications in July and is aimed at projects that can help NHS organisations in England support patients with long term conditions focusing on supporting people at home.
BioSpace
OCTOBER 4, 2021
AstraZeneca submitted data for an Emergency Use Authorization for AZD7442, a long-acting antibody combination from B-cells donated by patients who recovered from COVID-19.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
BioPharma Reporter
OCTOBER 4, 2021
While several vaccines protect against meningitis, there remains an urgent need for innovation, funding and research to develop more meningitis-preventive vaccines, according to a new report from the WHO.

BioSpace
OCTOBER 4, 2021
?The DOL's Office of Federal Contract Compliance Programs found that AstraZeneca paid a much lower base salary than similar employees to 23 Hispanic female staff and 295 female employees.

XTalks
OCTOBER 4, 2021
Nestlé Cereals, a UK division of the international food giant, recently announced the launch of Cheerios Vanilla O’s, a low-sugar cereal that contains no artificial colors or flavors. Classified as non-HFSS (high in fat, salt or sugar) under new UK government regulations , the new cereal’s main ingredient is whole grains. So, how does it stack up against its competitors and will it find success among a plethora of other better-for-you cereals?

BioSpace
OCTOBER 4, 2021
?Researchers at UCSF believed that a more personalized, deep brain stimulation could be a key. While DBS isn’t new, the UCSF team’s approach in their recently released study was unique.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Pharma Times
OCTOBER 4, 2021
The UK’s largest laboratory diagnostics company, Randox, has announced a nationwide expansion of ten new adaptive ‘cube’ laboratories across Great Britain, with facilities that provide a rapid and cost-effective model to expand laboratory provision.

pharmaphorum
OCTOBER 4, 2021
Episode 41 of the pharmaphorum podcast heard from Amit Nastik about the impact of COVID-19 on pharma supply chains and how he and his team mitigated disruption to Novartis’ own operations. Amit’s the global head strategy and operations and local markets manufacturing at Novartis Technical Operations and discussed how his company ensured an uninterrupted supply of medicines during COVID’s acute phase.

BioSpace
OCTOBER 4, 2021
Novartis-backed Exo Therapeutics closed out 2020 by completing a $25 million Series A financing round. Nearly 10 months later, the small molecule drug discovery and development company has earned more support from investors.
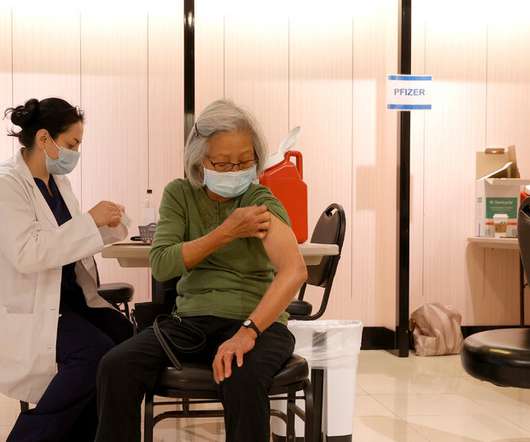
NY Times
OCTOBER 4, 2021
The request, expected this week, comes after a study found the company’s vaccine was only 71 percent effective against hospitalization from Covid-19.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Scienmag
OCTOBER 4, 2021
Automated vehicles could be made more pedestrian-friendly thanks to new research which could help them predict when people will cross the road. Credit: University of Leeds Automated vehicles could be made more pedestrian-friendly thanks to new research which could help them predict when people will cross the road. University of Leeds-led scientists investigating how to […].

NY Times
OCTOBER 4, 2021
The findings, which also document a waning of protection against infection, come as officials debate the necessity and timing of booster shots.

BioSpace
OCTOBER 4, 2021
Wanting to find out what is fueling the trend, BioSpace solicited the perspectives of a couple of executives who chose the SPAC route.
pharmaphorum
OCTOBER 4, 2021
Hard on the heels of impressive new data for Enhertu as a second-line therapy for breast cancer, AstraZeneca and Daichi Sankyo have claimed FDA breakthrough status for the drug that should shorten its review time. The new designation for Enhertu (trastuzumab deruxtecan) is based on the results of the DESTINY-Breast03 trial, which showed that the drug outperformed Roche’s rival therapy Kadcyla (trastuzumab emtansine) in HER2-positive breast cancer patients.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

BioSpace
OCTOBER 4, 2021
The ability to utilize one protein degrader against a whole host of diseases drives Kymera’s strategy when choosing protein targets.

Delveinsight
OCTOBER 4, 2021
Myocardial infarction is an irreversible death of heart muscles. It is pathologically referred to as myocardial cell death due to a prolonged lack of oxygen supply (ischemia). The condition is accompanied by chest pain that may appear as a sensation of tightness or pressure initially attributable to diminished cellular glycogen and relaxed myofibrils and sarcolemmal disruption that are a few of the first ultrastructural changes observed as early as 10–15 minutes after the onset of ischemia.

BioSpace
OCTOBER 4, 2021
With the planned departure from its 561,652-square-foot RTP campus, GSK will reduce its geographical footprint, but not its staff.
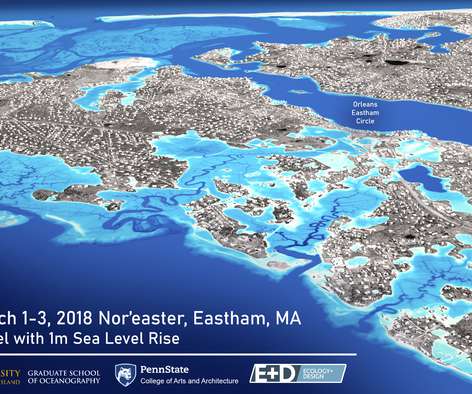
Scienmag
OCTOBER 4, 2021
KINGSTON, R.I. – Oct. 1, 2021 – Researchers at the University of Rhode Island and Penn State University have been awarded a four-year, $1.5 million grant through the National Oceanic and Atmospheric Administration to study the effects of sea level rise and how it may exacerbate the impact of extreme weather. The project will draw […].

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

BioSpace
OCTOBER 4, 2021
?Eli Lilly investigators said there might have been an error in the manufacturing process of lot D239382D but did not elaborate further.

pharmaphorum
OCTOBER 4, 2021
M3’s Tim Russell and Maxim Polyakov, together with Digital Futureway’s Heather Hancock, discuss new research on how doctor engagement has been altered by COVID-19. COVID-19 forced an unprecedented shift towards the virtual delivery of healthcare, suddenly compelling doctors increasingly to use digital means to care for their patients. As healthcare resources and delivery were prioritised, there was also a massive and ongoing impact on growing waiting lists and time-lags for diagnosis and treatme

BioSpace
OCTOBER 4, 2021
Henrietta Lacks’ family accuses Thermo Fisher Scientific of profiting off the first cell line that had been shared and replicated in a lab for the development of countless medical innovations.

NY Times
OCTOBER 4, 2021
Un número cada vez mayor de pacientes con cáncer, especialmente los de mama y pulmón, se libran del temido tratamiento en favor de otras alternativas.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content