Decentralized Clinical Trials in 2024: A Look Ahead
Crucial Data Soutions
DECEMBER 12, 2023
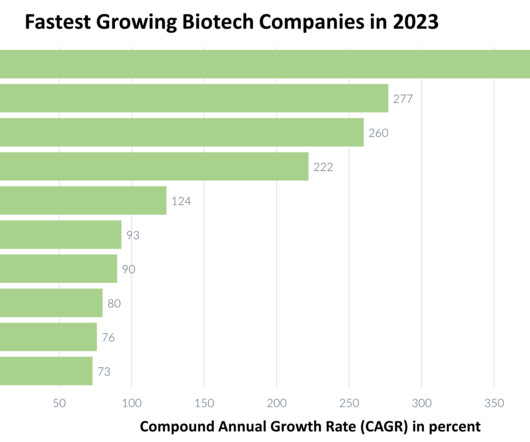
Decentralized clinical trials (DCTs) are growing. We may not be seeing the deep and widespread adoption of decentralized approaches some. The post Decentralized Clinical Trials in 2024: A Look Ahead appeared first on Crucial Data Solutions.







































Let's personalize your content