AstraZeneca and MSD’s Lynparza combo bags FDA approval for prostate cancer
Pharmaceutical Technology
JUNE 1, 2023
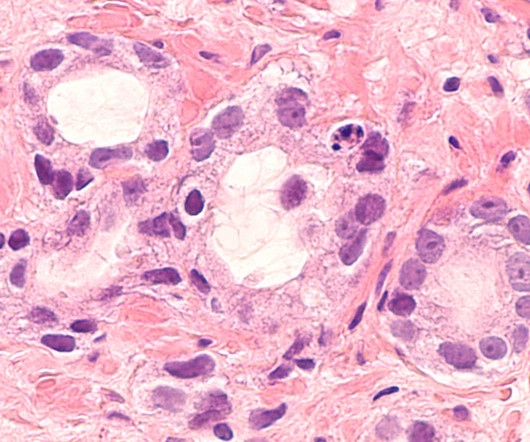
Safety and tolerability were in line with that observed in prior trials and the known profiles of the medicines. A prior orchiectomy or receipt of gonadotropin-releasing hormone (GnRH) analogs was also necessary. The PROpel study included 796 mCRPC patients who had not received prior chemotherapy or NHAs in the mCRPC setting.


































Let's personalize your content