New eBook: What does the future hold for gene editing & CRISPR?
Drug Discovery World
FEBRUARY 22, 2024
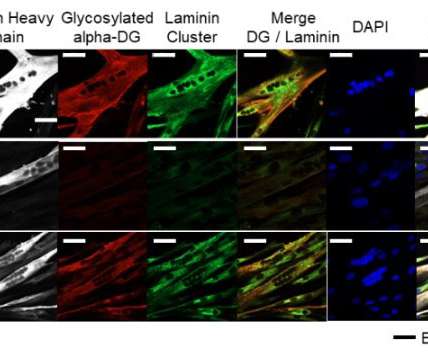
Following the first regulatory approval of a CRISPR-based drug in late 2023, over ten years since the CRISPR-Cas9 system was elucidated, there is considerable optimism about the future potential for gene editing technologies. appeared first on Drug Discovery World (DDW).









































Let's personalize your content