Vicore’s lung disease digital therapy gets breakthrough tag
pharmaphorum
MARCH 20, 2024
Vicore gets FDA breakthrough status for Almee, a cognitive behavioural therapy (CBT) digital health therapy used to support people with pulmonary fibrosis
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 tag disease
tag disease 
pharmaphorum
MARCH 20, 2024
Vicore gets FDA breakthrough status for Almee, a cognitive behavioural therapy (CBT) digital health therapy used to support people with pulmonary fibrosis

Pharmaceutical Technology
OCTOBER 17, 2023
The Netherlands-based company’s treatment has been awarded the designation by the US FDA following a successful Phase I trial.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
MARCH 20, 2024
Orchard Therapeutics has revealed the US price of Lenmeldy, its gene therapy for rare disease MLD, placing a $4.25m price tag on the one-shot treatment

Pharmaceutical Technology
SEPTEMBER 30, 2022
Skysona is indicated as a one-time gene therapy to slow the progression of cerebral adrenoleukodystrophy (CALD), a rare paediatric neurodegenerative disease in boys aged 4–17 years diagnosed with early-stage CALD. These approvals represent crucial milestones for bluebird bio, the gene therapy field, and patients with rare genetic diseases.

Fierce Pharma
JULY 14, 2023
The potentially curative promise of gene therapies often carries a steep price tag. | The potentially curative promise of gene therapies often carries a steep price tag. But for a pair of personalized medicine prospects in sickle cell disease (SCD), the cost could be worth it, at least as far as ICER is concerned.

Pharmaceutical Commerce
MARCH 6, 2024
Navigating the complex landscape of healthcare coverage can be an intimidating task, especially for patients with rare diseases for which treatments often come with a high price tag.

Fierce Pharma
NOVEMBER 7, 2023
Two long-awaited treatments for sickle cell disease (SCD) are on the docket for FDA decisions next month. Two long-awaited treatments for sickle cell disease (SCD) are on the docket for FDA decisions next month. Vertex and CRISPR Therapeutics are up first with a Dec. |
Pharmaceutical Technology
MAY 19, 2023
Multiple sclerosis (MS) is a primary autoimmune disease in which inflammation is a core contributor to the degeneration of the central nervous system (CNS), leading to neurological disability and affecting sensory, visual, motor, and autonomic systems. DMTs for MS have a high price tag, particularly in the US.

World of DTC Marketing
JUNE 1, 2021
Still, Alzheimer’s is a nasty disease, and people are desperate for treatment even if it only provided a slim chance of partial recovery. If the drug is approved you can bet that it’s going to carry a huge price tag. Will they follow the science or will they put a price tag on hope?

Drug Discovery World
OCTOBER 26, 2022
A new method to study the proteins released by cells, which could lead to new biomarkers for diseases including cancer, has been developed by scientists at the Francis Crick Institute and Imperial College London. . Biomarkers are valuable tools in diagnosing disease or predicting treatment outcomes, but they are challenging to find. .

STAT News
JULY 14, 2022
These treatments constitute a highly lucrative market because they are used to help the body fight cancer, infection, and a wide array of other diseases — and often carry considerable price tags. Continue to STAT+ to read the full story…

Rethinking Clinical Trials
JANUARY 23, 2024
People with angina-like symptoms are often not patients with a disease. Most do not have obstructive coronary artery disease (CAD), but a few are very high risk. They are trying to follow the guidelines for symptoms and disease. The tests are so common in aggregate it is a big expense. They are pretty good.
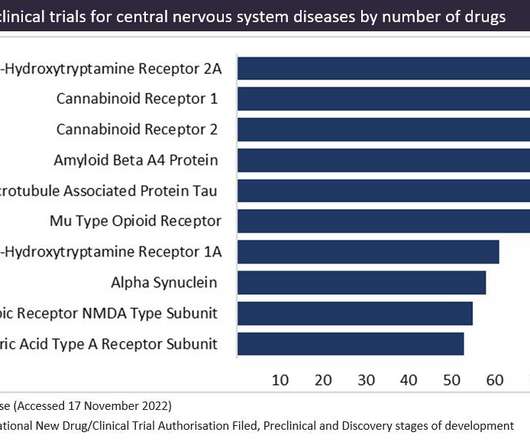
Pharmaceutical Technology
DECEMBER 5, 2022
Figure 1 shows the targets in preclinical trials for central nervous system diseases, as sourced from GlobalData’s Drugs Database. CB1 receptors are the joint first most popular target with serotonin receptor 2A in preclinical trials for central nervous system diseases. This is closely followed by CB2 receptors in second place.

Pharmaceutical Technology
JUNE 29, 2022
This year has already been eventful when it comes to the development of therapies for rare diseases. Additionally, pricing and access for rare disease therapies continue to be scrutinized closely. Others judge its success based on the fact that 95% of rare diseases still have no available therapies and patient needs remain unmet.

Scienmag
MARCH 16, 2021
Multidisciplinary project will use dendritic tags to enable food traceability any point in the supply chain Credit: Northern Arizona University According to the Centers for Disease Control and Prevention, more than 48 million Americans are sickened by foodborne illnesses each year, costing the economy more than $15 billion.

Sciensano
MAY 18, 2022
The objective of this project was to implement a multidisciplinary approach and create a network of experts in order to improve the surveillance of infectious diseases with the help of cutting-edge technologies, such as high-throughput sequencing. BE READY project coordinator, Transversal activities in Applied Genomics ( TAG )).
STAT News
OCTOBER 6, 2022
… Multimillion-dollar prices for a rival’s treatments with the potential to cure rare diseases are “in the right ballpark,” according to Stuart Arbuckle, chief operating officer at Vertex Pharmaceuticals, which is preparing to market its own treatment for sickle-cell disease , Bloomberg News tells us. Even
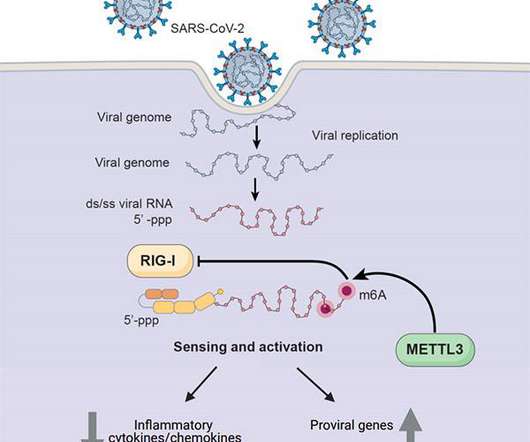
Scienmag
APRIL 29, 2021
Credit: UC San Diego Health Sciences Researchers at University of California San Diego School of Medicine have discovered one way in which SARS-CoV-2, the coronavirus that causes COVID-19, hijacks human cell machinery to blunt the immune response, allowing it to establish infection, replicate and cause disease.
World of DTC Marketing
JULY 20, 2022
Pfizer, for instance, hiked the cost of its leukemia medication Besponsa again this month, bringing its per-vial price tag to $21,056. The patient group also spotlighted AAmgen’sprice hikes for its autoimmune disease drug Enbrel. inflation rate.
pharmaphorum
DECEMBER 24, 2021
The anti-amyloid drug, which also claimed a breakthrough tag from the FDA in July, is being developed for the treatment of early-stage Alzheimer’s disease. The $56,000-a-year price tag for the drug, which has now been cut in half in the US, also hasn’t helped the drugmakers make a case for Aduhelm with US payers.

Pharmaceutical Technology
JULY 14, 2022
It is estimated that there are currently more than 7,000 orphan diseases, many of which are considered life-threatening and most of which have a genetic basis. Despite this high number, orphan diseases are rare by definition, affecting around one in 2,000 people as defined by the European Union. Go-to-market strategies.

Pharmaceutical Technology
APRIL 13, 2023
Two gene therapies up for approval this year for sickle cell disease could be cost effective in some cases at a $2 million price point, based on a draft evidence report published by the Institute for Clinical and Economic Review (ICER). The standard of care for the condition includes blood transfusions, hydroxyurea and iron chelation.
Drug Discovery World
NOVEMBER 28, 2023
An experimental drug has shown potential as a disease-modifying therapy for Parkinson’s disease, according to a new study published in Nature Communications. Parkinson’s disease is highly associated with mitochondrial dysfunction. Mission is planning to initiate a MTX325 Phase I trial in humans in early 2024.

XTalks
APRIL 18, 2024
This test, which is being co-developed with Eli Lilly, has the potential to facilitate earlier and more accurate detection of the disease. The Elecsys pTau217 assay is designed to detect amyloid pathology, a hallmark of Alzheimer’s disease, by measuring phosphorylated tau protein in human plasma.

pharmaphorum
OCTOBER 18, 2022
Medtech giant Beckton Dickinson (BD) has signed a deal with France’s Biocorp to use the latter’s near-field communication (NFC) tags in injectable devices. The Injay tag can confirm a complete injection and transfer that information via an NFC reader to a smartphone or tablet for review by a healthcare professional.

Rethinking Clinical Trials
FEBRUARY 13, 2023
All infants and children less than 2 years of age undergoing open heart surgery on cardiopulmonary bypass were eligible for the study with exclusions for diseases that implied high severity pre-bypass. One of the main challenges of cardiac surgery, in addition to the technical complexities, is the occurrence of low cardiac output syndrome.

Rethinking Clinical Trials
AUGUST 30, 2023
Speaker Gabriela Schmajuk, MD, MS Professor of Medicine UCSF and the San Francisco VA Slides Keywords Patient-Reported Outcomes, Rheumatoid Arthritis, EHR Key Points Clinicians rely on patient-related outcomes (PROs) to track disease and function over time in patients with rheumatoid arthritis (RA).
Pharmaceutical Technology
FEBRUARY 10, 2023
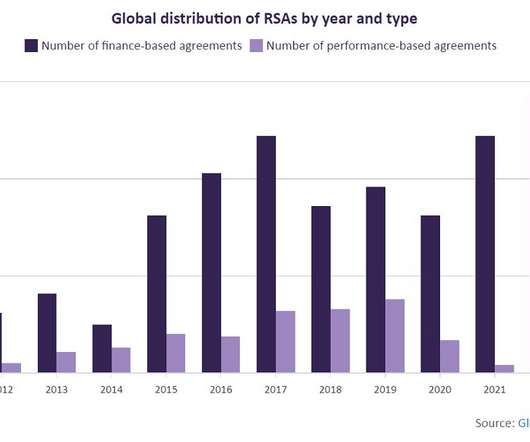
This is not the first treatment to come with a high price tag. However, more highly innovative/high-cost treatments, within infectious disease and neurology space, are likely to enter the market for rare disorders, increasing the number of non-oncology RSAs.

World of DTC Marketing
NOVEMBER 3, 2020
Cardiovascular disease deaths in the US have increased by nearly 17% since 2010, and are among the reasons that life expectancy in the U.S. The Global Burden of Disease study estimates that high sodium intake causes between 1 million and 5 million deaths per year globally” Then there is the obesity epidemic.

XTalks
MARCH 25, 2024
Cocoa trees, highly sensitive to climate variations, are struggling with disease due to last season’s heavy rainfall. For consumers, the joy of indulging in Easter chocolates may come with a higher price tag, prompting a reflection on the sustainability and future of this beloved treat.

Pharmaceutical Technology
MAY 2, 2023
While initially only approved for metastatic disease, ICIs have now moved into earlier disease settings, reducing the risk of disease progression and relapse. IO therapies come with a hefty price tag, with ICI therapies in the US typically exceeding $100,000, while cell therapies can exceed $400,000.

The Pharma Data
NOVEMBER 29, 2021
1.1.529 a variant of concern, named Omicron, on the advice of WHO’s Technical Advisory Group on Virus Evolution (TAG-VE). Severity of disease : It is not yet clear whether infection with Omicron causes more severe disease compared to infections with other variants, including Delta. Recommended actions for countries.

XTalks
DECEMBER 13, 2023
The US Food and Drug Administration (FDA) has approved the first gene therapies for the treatment of sickle cell disease, approving two on the same day. Both gene therapies are approved for individuals 12 years of age and older with sickle cell disease. Casgevy is also the first ever CRISPR/Cas9-based therapy approved in the US.

XTalks
FEBRUARY 27, 2024
Rare Disease Day 2024, which falls on February 29 this year, is an opportunity to unite under a common cause: to bring attention to the challenges faced by those living with rare diseases and to push for advancements in research, treatment and policy. In 2020, 31 out of 53 novel drug approvals were for rare or orphan diseases.

XTalks
AUGUST 19, 2022
But bluebird bio has said such setbacks are expected for a company at the frontier of developing gene therapies for rare diseases. The most severe form of the disease is sometimes called transfusion-dependent beta-thalassemia or beta-thalassemia major. Zynteglo’s hefty price tag of $2.8

Rethinking Clinical Trials
JANUARY 17, 2024
Tags #pctGR, @Collaboratory1 The post Grand Rounds January 12, 2024: Design and Implementation of a Weighted Lottery to Equitably Allocate Scarce Covid-19 Resources (Erin K. Speaker Erin K. We know there is a digital gap in disadvantaged patients and elderly patients. Many patients did not answer the phone.

Rethinking Clinical Trials
APRIL 18, 2023
People with experience to prior disease that might be similar is where we started. Tags #pctGR, @Collaboratory1 The post Grand Rounds April 14, 2023: RECOVER in Action – Status of Clinical Trial Protocols (Kanecia Zimmerman, PhD, MD, MPH) appeared first on Rethinking Clinical Trials.

Rethinking Clinical Trials
DECEMBER 20, 2023
Globally cardiovascular disease (CVD) prevalence had decreased between 1990 and 2010, but it has slightly increased since 2010. Tags #pctGR, @Collaboratory1 The post Grand Rounds December 15, 2023: Diversifying Clinical Trials: A Path Forward (Roxana Mehran, MD, FACC, FAHA, MSCAI, FESC) appeared first on Rethinking Clinical Trials.

Rethinking Clinical Trials
JANUARY 26, 2023
The treatment is on-label because anyone with an acute coronary has at risk cardiovascular disease so technically it is on-label. Tags #pctGR, @Collaboratory1 The post Grand Rounds January 20, 2023: Collaborative Pragmatic Trials in Action: EVOLVE-MI (Mikhail Kosiborod, MD) appeared first on Rethinking Clinical Trials.

World of DTC Marketing
SEPTEMBER 9, 2021
A study put a price tag on American’s bad eating habits: $50 billion a year in health care costs, attributable to cardiometabolic diseases such as heart disease, stroke, and type 2 diabetes. Then there is the cost of unhealthy Americans.

The Pharma Data
FEBRUARY 22, 2022
As part of its on-going work to track variants, WHO’s Technical Advisory Group on SARS-CoV-2 Virus Evolution ( TAG-VE ) met yesterday to discuss the latest evidence on the Omicron variant of concern, including its sublineages BA.1 2 may cause more severe disease in hamsters compared to BA.1. Reinfection with BA.2

Rethinking Clinical Trials
OCTOBER 11, 2023
Murray, PhD NIH Associate Director for Prevention and Director, NIH Office of Disease Prevention Moderator: Jonathan C. Moyer, PhD Statistician, NIH Office of Disease Prevention Slides Keywords Implementation; Study design; Hybrid; Clustered; DECIPHeR Key Points People often contest that hybrid designs are not as rigorous as they should be.

pharmaphorum
APRIL 26, 2021
The initiative was supported by grants from UCB and Pfizer and provided health information in the form of immersive experiences aimed at patients prescribed biologic drugs to treat the disease. The company halted development before it eventually sold its digital health unit back to Eric Careel, co-founder of Withings, the following year.

pharmaphorum
JANUARY 18, 2022
Two Spanish pharma companies are joining forces to identify new oral treatments for immune-inflammatory skin diseases with high unmet medical needs. Almirall and IRB Barcelona (the Institute for Research in Biomedicine), will conduct research using molecular glue degraders, a new approach to the skin diseases under scrutiny.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content