Smart pharmaceutical and healthcare labels: Lets trace medicines from its origin
Roots Analysis
MARCH 8, 2023
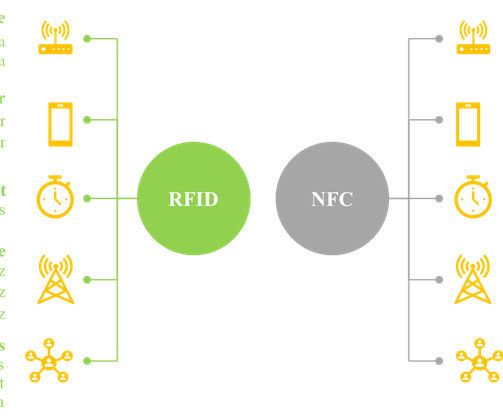
In recent years, the use of smart labels allows the developer to convey a greater amount of information about the product to the consumers, without the need for additional packaging space. While most smartphones can read NFC chips, RFID tags can only be read by specialized receivers.













































Let's personalize your content