NorthSea Therapeutics bags FDA rare paediatric disease tag for NASH drug
Pharmaceutical Technology
OCTOBER 17, 2023
The Netherlands-based company’s treatment has been awarded the designation by the US FDA following a successful Phase I trial.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 tag therapeutics
tag therapeutics 
Pharmaceutical Technology
OCTOBER 17, 2023
The Netherlands-based company’s treatment has been awarded the designation by the US FDA following a successful Phase I trial.

pharmaphorum
MARCH 20, 2024
Orchard Therapeutics has revealed the US price of Lenmeldy, its gene therapy for rare disease MLD, placing a $4.25m price tag on the one-shot treatment
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
SEPTEMBER 30, 2022
Spark Therapeutics’ Luxturna, indicated for inherited retinal disease (IRD), was the first gene therapy to be approved, in 2017, with a price tag of $850,000 for each eye. Gene therapies for rare inherited diseases are very expensive, as they tend to be the only therapeutic option available to patients and are often curative.

Fierce Pharma
NOVEMBER 7, 2023
Vertex and CRISPR Therapeutics are up first with a Dec. | But on Tuesday bluebird said that Vertex’s price tag will not factor into how the Massachusetts company will price its treatment. Two long-awaited treatments for sickle cell disease (SCD) are on the docket for FDA decisions next month.
Pharmaceutical Technology
DECEMBER 15, 2022
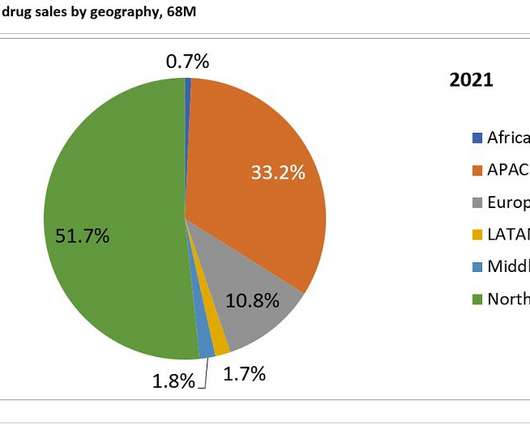
Prior to the development of Camzyos and aficamten, obstructive HCM has not been the target of clinical trial development, so these two therapeutics would benefit a neglected patient population. of sales in 2021 and 2031 respectively. The second highest contributing market is APAC, generating 33.2%

Rethinking Clinical Trials
JANUARY 17, 2024
The COVID Therapeutics Committee worked with the state health department to develop a policy for fair allocation of scarce medications to treat COVID-19. The COVID Therapeutics Committee created communication materials to be transparent and to get buy-in. All eligible patients were gathered from data warehouse and manual EHR entry.
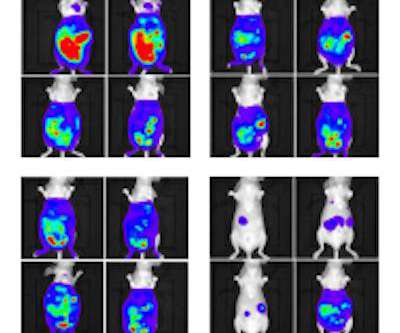
Scienmag
APRIL 23, 2021
Credit: RIKEN Researchers led by Katsunori Tanaka and Kenward Vong at the RIKEN Cluster for Pioneering Research (CPR) in Japan have demonstrated that tumor growth can be reduced by therapy that tags cancer cells with different therapeutic molecules.

Rethinking Clinical Trials
JULY 22, 2022
There were four ACTIV fast-track focus areas: vaccines, preclinical, clinical trial capacity, and therapeutics – clinical. In the therapeutics arm, the most promising therapeutic agents for COVID-19 were prioritized. Read more in Annals of Internal Medicine and Critical Care Medicine. pctGR, @Collaboratory1.

Rethinking Clinical Trials
APRIL 18, 2023
This broad set of clinical conditions and varied underlying causes underscore the need for testing a broad portfolio of therapeutic agents. RECOVER is a patient-centered, integrated, adaptive research network. It seems the most severely affected people came from the early waves of COVID.

Pharmaceutical Technology
MAY 2, 2023
Immuno-oncology (IO) agents have transformed the cancer therapeutics landscape, driving long-term remissions in a subset of patients who historically had limited options. IO agents include the classes of immune checkpoint modulators, cell therapies, bispecific antibodies, oncolytic viruses, therapeutic vaccines, and cytokines.
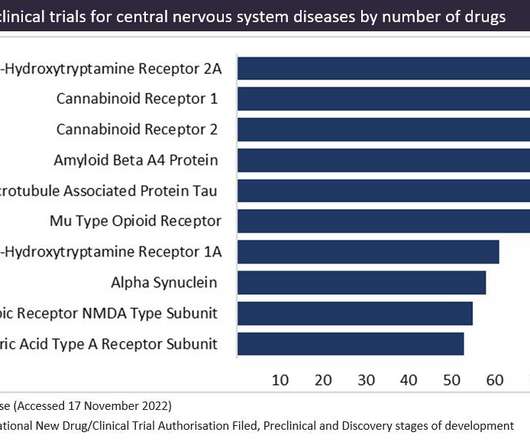
Pharmaceutical Technology
DECEMBER 5, 2022
Cannabinoid receptors are a popular therapeutic target for cannabinoid-based drugs in the treatment of pain, neurological disorders and inflammation, according to GlobalData’s Pharma Intelligence Centre Drugs database. This is closely followed by CB2 receptors in second place.
Drug Discovery World
NOVEMBER 28, 2023
The peer-reviewed article titled ‘Knockout or inhibition of USP30 protects dopaminergic neurons in a Parkinson’s disease (PD) mouse model’ was the result of collaborative work between Cambridge University, Harvard Medical School, and Mission Therapeutics. Parkinson’s disease is highly associated with mitochondrial dysfunction.

pharmaphorum
APRIL 26, 2021
Technology firm Jolly Good and Teijin Pharma have begun a partnership to develop virtual reality digital therapeutics (VR DTx) for major depressive disorder. However Nokia famously ran into trouble when it tried to develop VR products for digital health and its OZO camera failed to catch on, mainly because of a high $60,000 price tag.

The Pharma Data
FEBRUARY 22, 2022
As part of its on-going work to track variants, WHO’s Technical Advisory Group on SARS-CoV-2 Virus Evolution ( TAG-VE ) met yesterday to discuss the latest evidence on the Omicron variant of concern, including its sublineages BA.1 Reinfection with BA.2 2 following infection with BA.1 WHO will continue to closely monitor the BA.2
Pharmaceutical Technology
MAY 15, 2023
The pharmaceutical industry continues to be a hotbed of innovation, with activity driven by the evolution of new treatment paradigms, and the gravity of unmet needs, as well as the growing importance of technologies such as pharmacogenomics, digital therapeutics, and artificial intelligence.
Drug Discovery World
JANUARY 9, 2023
Using gene engineering, we are repurposing cancer cells to develop a therapeutic that kills tumour cells and stimulates the immune system to both destroy primary tumors and prevent cancer.” . The researchers also built a two-layered safety switch into the cancer cell, which can eradicate the therapeutic tumour cells if needed.
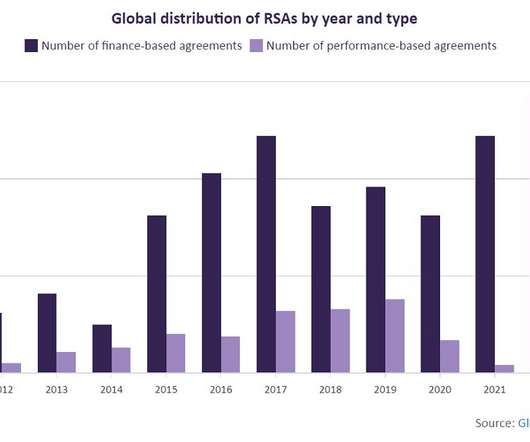
Pharmaceutical Technology
FEBRUARY 10, 2023
This is not the first treatment to come with a high price tag. Because RSAs traditionally involve high cost/highly innovative drugs, GlobalData has reviewed the top brands according to volume of RSAs granted and Anatomical Therapeutic Chemical (ATC) code.

Pharmaceutical Technology
JUNE 29, 2022
For example, while orphan drug designations have more than doubled in the last decade compared to the previous one, only 16% of therapies with orphan tags have managed to gain FDA approval in some indications. During 2001–10, the number of granted designations almost tripled, with 1,527 drugs receiving their first orphan drug designation.

XTalks
AUGUST 31, 2022
In this episode, Ayesha discussed the FDA approval of Axsome Therapeutics’ rapid-acting oral treatment Auvelity for the treatment of major depressive disorder (MDD). Hear more about the therapy, some of the setbacks bluebird has had to face on the road to its approval and why the treatment has a steep price tag. Bluebird’s $2.8M

pharmaphorum
JANUARY 11, 2022
A prescription digital therapeutic (DTx) for leukaemia patients developed by Blue Note Therapeutics has been awarded breakthrough device status by the FDA. The post Blue Note leukaemia DTx gets FDA breakthrough tag appeared first on. Other DTxs are aimed at people being treated for breast and lung cancers.
Drug Discovery World
OCTOBER 31, 2022
The last month has seen huge strides forward in our understanding of cancers, particularly in how they develop resistance to therapies and how we can ‘outsmart’ them using gene editing or different therapeutic pathways, but also how we can better target drugs to individuals and accurately predict treatment outcomes.

World of DTC Marketing
SEPTEMBER 7, 2022
In pharma, growth depends on new products with hefty price tags when over 80% of voters want lower costs for their prescription drugs. oncology therapeutics market has reached $71 billion. Wall Street wants growth, as do investors, but that may be impossible for pharmaceutical companies. ” The U.S.

pharmaphorum
JANUARY 18, 2022
The use of monovalent degraders, which enable the targeted degradation of disease-relevant proteins, offers a new avenue to reach tissue that cannot be targeted with conventional therapeutic agents. Researchers at Almirall have identified several proteins whose abnormal function is associated with inflammatory immune skin diseases.

XTalks
JANUARY 28, 2022
Kimmtrak has a price tag of $18,760 per vial, which amounts to a weekly dose. Kimmtrak is part of a novel class of bispecific T cell immunotherapies being developed by Immunocore. Kimmtrak has also become the first bispecific T cell engager to be FDA-approved for the treatment of a solid tumor.
pharmaphorum
NOVEMBER 24, 2022
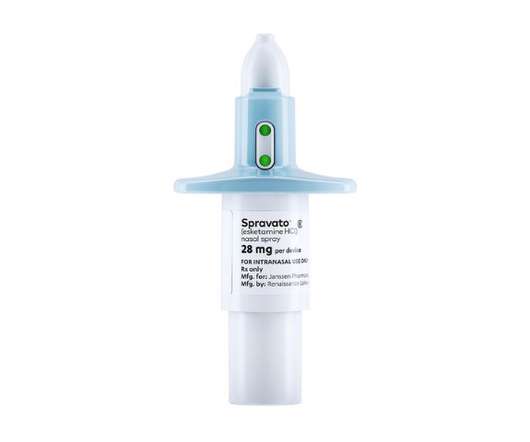
One issue has been the cost of the drug, with health technology assessment agency NICE in the UK and the ICER organisation in the US both concluding its price tag means it is not a cost-effective option for health systems. The post J&J builds case for antidepressant Spravato with head-to-head trial appeared first on.

Pharmaceutical Technology
APRIL 13, 2023
Released on April 12, the report focuses on bluebird bio’s lovotibeglogene autotemcel and Vertex Pharmaceuticals and CRISPR Therapeutics’ exagamglogene autotemcel or exa-cel and their potential use in treating sickle cell disease.

The Pharma Data
AUGUST 18, 2020
Bristol Myers Squibb has scooped up the sole rights to the interleukin-12 (IL-12) immunotherapy programme of Massachusetts-based biotech Dragonfly Therapeutics, handing over $475 million for the privilege.

The Pharma Data
NOVEMBER 29, 2021
1.1.529 a variant of concern, named Omicron, on the advice of WHO’s Technical Advisory Group on Virus Evolution (TAG-VE). WHO’s TAG-VE will continue to monitor and evaluate the data as it becomes available and assess how mutations in Omicron alter the behaviour of the virus. Recommended actions for countries.

Pharmaceutical Technology
FEBRUARY 22, 2023
Both drugs come with a high price tag. On the opposing side, Sanofi argues that Amgen’s approach not only violates enablement requirements, but also stifles innovation by stopping others from developing new therapeutics, says Falati. Both drugs are monoclonal antibodies that inhibit the protein PCSK9.

pharmaphorum
NOVEMBER 24, 2021
Digital health company Pear Therapeutics has won FDA breakthrough device status for reSET-A, its development-stage prescription digital therapeutic (DTx) for people with alcohol-use disorder. The post Pear claims breakthrough tag for alcohol use disorder DTx appeared first on. Photo by thom masat on Unsplash.

Rethinking Clinical Trials
DECEMBER 14, 2022
During the Ebola crisis, the ethical argument for stepped wedge CRTs was that anything involving a placebo in the control arm was automatically considered unethical if the intervention arm holds a chance of benefit. It’s still important to look at how effective it is and is the juice worth the squeeze in this context? pctGR, @Collaboratory1.

The Pharma Data
JANUARY 31, 2021
Utilizing Clover’s proprietary Trimer-Tag© technology, S-Trimer is a trimeric SARS-CoV-2 spike (S)-protein subunit vaccine candidate. About Trimer-Tag© Technology. Trimer-Tag© is an innovative drug development platform which allows the production of novel, covalently-trimerized fusion proteins. —- End —-.

XTalks
DECEMBER 13, 2023
The landmark approvals were awarded to bluebird bio’s Lyfgenia (lovo-cel) and Vertex Pharmaceuticals and CRISPR Therapeutics’ jointly developed Casgevy (exa-cel). The revolutionary CRISPR/Cas9 technology was discovered by Jennifer Doudna and CRISPR Therapeutics co-founder Emmanuelle Charpentier who both shared the 2020 Nobel Prize for it.

Rethinking Clinical Trials
MARCH 28, 2023
across all therapeutic areas. Every PCORnet®. project has very robust approaches to engaging patients across the continuum of the research from the identification of research questions to the planning phase to the implementation phase to the analysis phase and out to the dissemination of results.
pharmaphorum
JUNE 12, 2022
Beti-cel has already been approved for marketing in Europe as Zynteglo, with a price tag of around $1.8 million price tag, saying its ability to help patients reach sustained transfusion independence justified its high price. Eli-cel was also previously approved in Europe as Skysona.

pharmaphorum
AUGUST 21, 2022
Shares in Axsome Therapeutics have rocketed on FDA approval of its depression therapy Auvelity (formerly AXS-05) – a year after its approval was held up by the regulator. Auvelity is the first drug Axsome has taken through to regulatory approval, but could be the first of a string of new products for the company, according to Tabuteau.

Rethinking Clinical Trials
OCTOBER 27, 2022
Should social media for teenagers be regulated like a digital therapeutic, given how addictive they are and the behavior modification. Case study: The U.K. The Light Collective is an organization that represents collective rights, interests, and voices of patient communities in healthcare technology. pctGR, @Collaboratory1.
pharmaphorum
OCTOBER 21, 2021
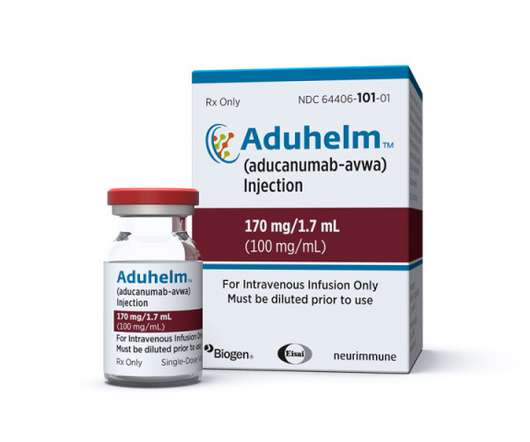
Vounatsos also insisted that Aduhelm’s price tag has not been a factor discouraging treatment with the drug. The slow roll out of Aduhelm raises pressure on Biogen to get the most out of Vumerity and other drugs like Sage Therapeutics-partnered antidepressant zuranolone, which is due to be filed next year.

pharmaphorum
APRIL 5, 2022
Immunocore now has approval on both side of the Atlantic for Kimmtrak – the first cancer therapeutic based on T cell receptor (TCR) technology – after getting a green light from the European Commission. The European population is meanwhile estimated at several thousand patients, across all stages, according to registry data.

The Pharma Data
DECEMBER 3, 2020
Utilizing Clover’s proprietary Trimer-Tag © technology, S-Trimer is a trimeric SARS-CoV-2 spike (S)-protein subunit vaccine candidate. About Trimer-Tag © Technology. Trimer-Tag © is an innovative drug development platform which allows the production of novel, covalently-trimerized fusion proteins.

Drug Discovery World
JANUARY 30, 2024
He said: “The industry, as a whole, needs to carefully consider the true value of curing lifelong genetic or degenerative diseases, particularly when evaluating novel therapeutics that present unique treatment opportunities.” Pricing and reimbursement continues to be a challenge we address as a field.

The Pharma Data
MARCH 16, 2022
using SNAP-tag) involves labeling cell proteins with a fluorescent dye, thereby enabling researchers to observe how proteins interact in the living cell and where in the cell they are located. With Spirochrome, he commercialized fluorescent probes for cell biology, while with Covalys, he marketed SNAP-tag and other protein labeling methods.

Delveinsight
FEBRUARY 9, 2021
Deerfield Management has entered into a research agreement with Dana-Farber Cancer Institute to fuel the development of therapeutics and diagnostics for cancer. TG Therapeutics Wins FDA Nod for Ukoniq for Marginal Zone Lymphoma and Follicular Lymphoma. TG Therapeutics has announced the U.S.

The Pharma Data
JANUARY 31, 2021
Utilizing Clover’s proprietary Trimer-Tag© technology, S-Trimer is a trimeric SARS-CoV-2 spike (S)-protein subunit vaccine candidate. About Trimer-Tag© Technology. Trimer-Tag© is an innovative drug development platform which allows the production of novel, covalently-trimerized fusion proteins. . EMERYVILLE, Calif.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content