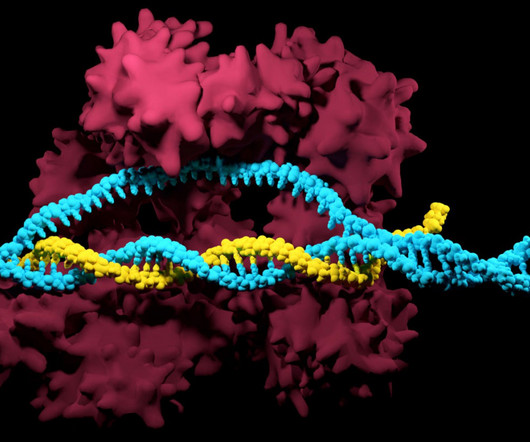
Leading innovators in transcription factors for genetically modified cells for the pharmaceutical industry
Pharmaceutical Technology
JUNE 4, 2023
In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Innovation in Pharmaceuticals: Transcription factors for genetically modified cells. However, not all innovations are equal and nor do they follow a constant upward trend.









































Let's personalize your content