Early data shows Russia’s Sputnik V COVID-19 vaccine produces immune response
pharmaphorum
SEPTEMBER 7, 2020
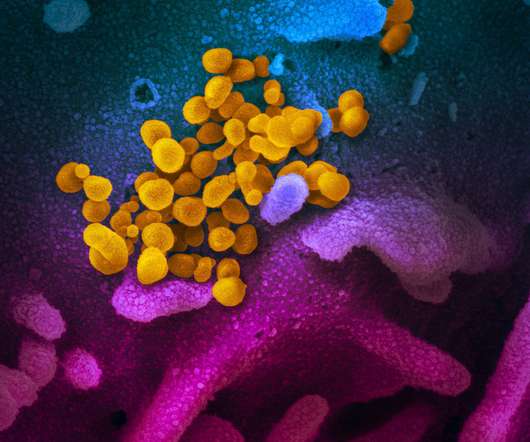
A trial of Russia’s Sputnik V coronavirus vaccine has shown the jab produces an immune response, although the study was too small to produce conclusive findings, particularly on safety. All participants in the phase 2 trials (40 participants) produced antibodies against the SARS-CoV-2 spike protein.







































Let's personalize your content