Konvomep Gets FDA Approval as a New Oral Liquid Formulation Option of Omeprazole
XTalks
SEPTEMBER 6, 2022
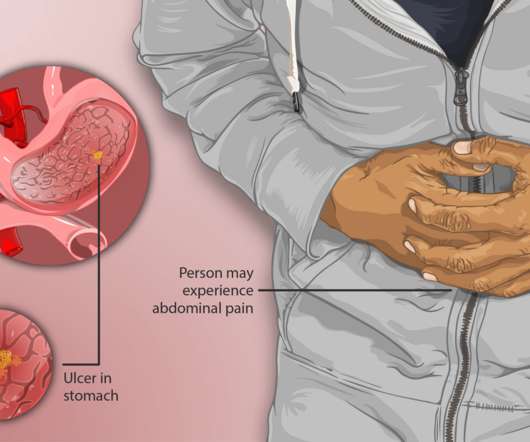
Other factors like frequently consuming alcohol or using steroids often may also increase the chance of developing a gastric ulcer. Doctors may prescribe a proton pump inhibitor (PPI), such as omeprazole, which blocks acid-producing cells to protect the gastric ulcer from acid while it heals naturally.




































Let's personalize your content