General Design Methods for mRNA Drugs
Pharma Mirror
APRIL 21, 2024
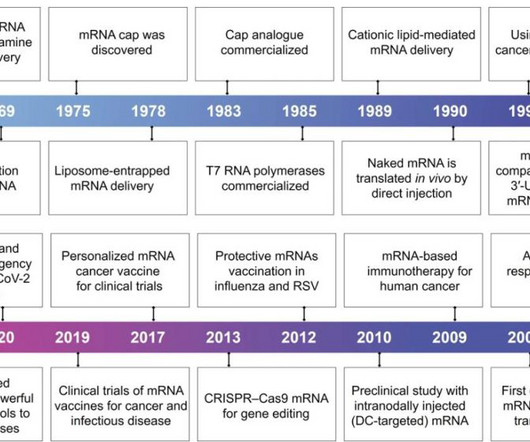
With the rapid development of biotechnology and molecular medicine, the introduction of mRNA as a vaccine or therapeutic agent enables the production of almost any desired functional protein/peptide within the human body.



































Let's personalize your content