After slower-than-expected review, Pfizer and Arena to close buyout deal
Bio Pharma Dive
MARCH 10, 2022
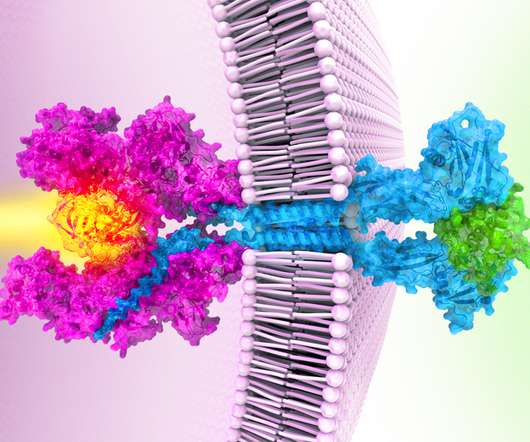
Arena Pharmaceuticals expects its $7 billion sale to Pfizer to close Friday, ending a slightly protracted regulatory review that raised questions about whether the deal would be cleared as anticipated.





































Let's personalize your content