Cannabinoids receptors: popular preclinical target but banned in 137 countries
Pharmaceutical Technology
DECEMBER 5, 2022
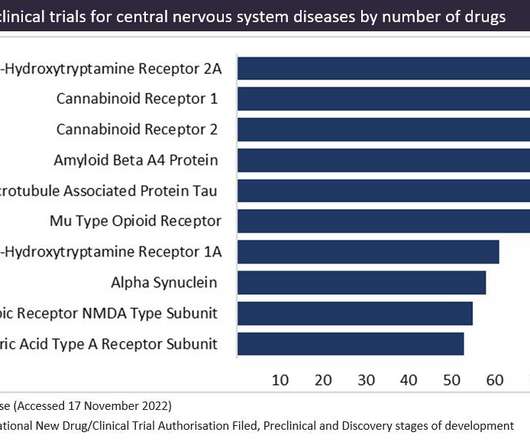
Despite this, the medical use of cannabinoid drugs is heavily restricted, including being banned in 137 countries, according to the United Nations. Collectively, cannabinoid receptors (CB1 and CB2) are currently the most popular targets in preclinical stage of development, with 391 drugs tagged in total.














































Let's personalize your content