Sarah Wicks Returns to HPM to Elevate Drug Development and Regulatory Practice
FDA Law Blog
MAY 1, 2024
Sarah brings a wealth of experience and a proven track record of advising innovative drug and biologics companies through the intricate landscape of product development and commercialization. Sarah’s a tremendous attorney who always understood HPM’s culture and commitment to client service. I am very excited to have Sarah rejoin HPM.





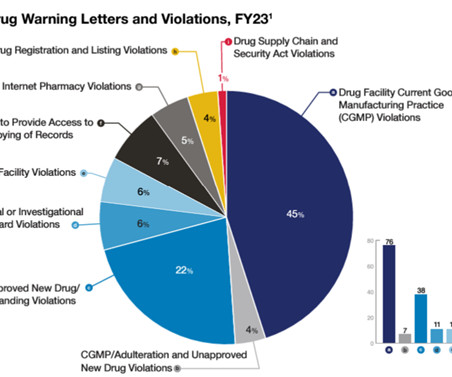







































Let's personalize your content