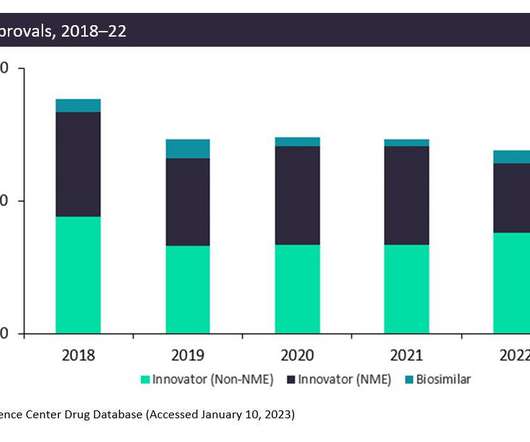
In a first, FDA approves an 'interchangeable' biosimilar for diabetes
Bio Pharma Dive
JULY 29, 2021
An injectable insulin from Viatris has become the first-ever biosimilar product that can be directly substituted for a marketed biologic, a long-awaited decision that could put pricing pressure on other diabetes drugs.










































Let's personalize your content