Smart pharmaceutical and healthcare labels: Lets trace medicines from its origin
Roots Analysis
MARCH 8, 2023
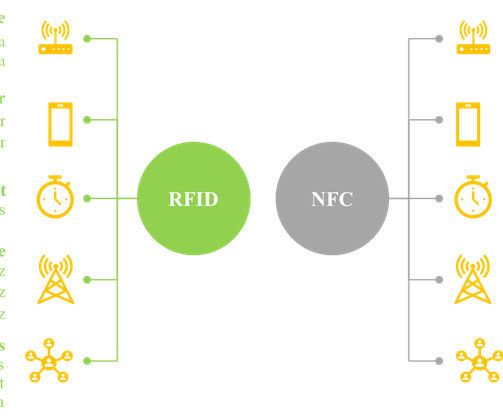
It is worth noting that smart labels contain a transponder code which can be read by sophisticated devices, including radio frequency identification device (RFID) tags and near-field communication (NFC) chips. While most smartphones can read NFC chips, RFID tags can only be read by specialized receivers.

































Let's personalize your content