Subcutaneous Medications and Drug Delivery Systems
Roots Analysis
FEBRUARY 26, 2024
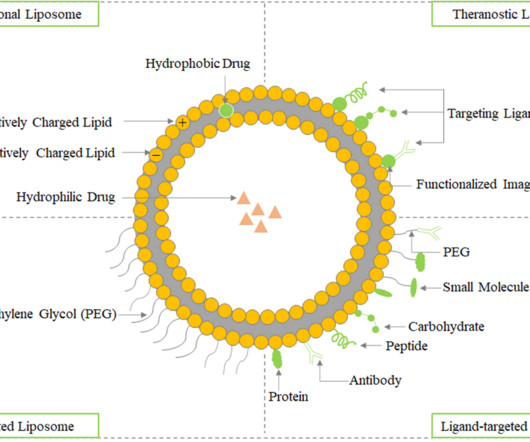
Given the robust pipeline of biologics, which include monoclonal antibodies, vaccines and other protein-based therapeutic products, subcutaneous medications are being investigated for various clinical candidates across different phases of development.








































Let's personalize your content