CDSCO empowers regulators & boosts exports through comprehensive training programmes
AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 2, 2024
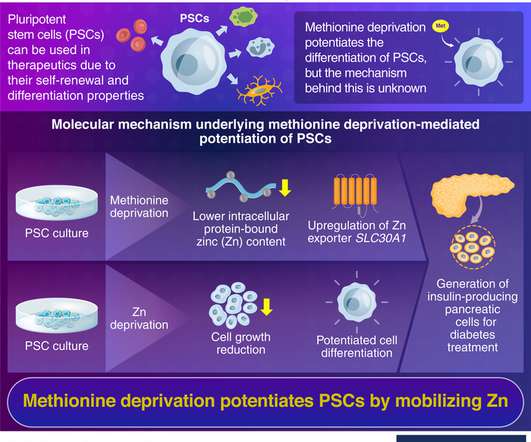
With a focus on equipping regulators with advanced skills, the CDSCO conducted 23 residential training programmes tailored to address critical aspects such as […]









































Let's personalize your content