FDA accepts AstraZeneca’s NDA for breast cancer combination therapy
Pharmaceutical Technology
JUNE 13, 2023
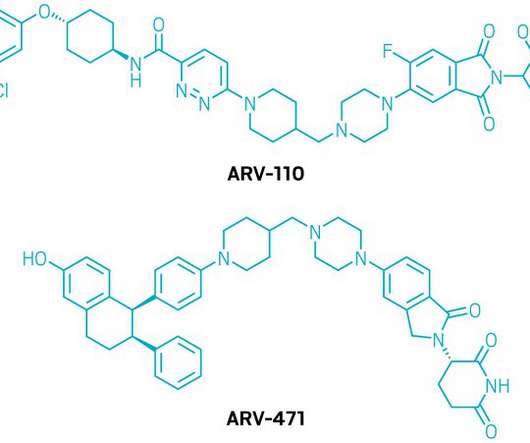
The US Food and Drug Administration (FDA) has accepted AstraZeneca’s new drug application (NDA) for the combination of capivasertib and FASLODEX (fulvestrant), and granted it priority review. The submission of the NDA was based on the findings of the CAPItello-291 Phase III trial.









































Let's personalize your content